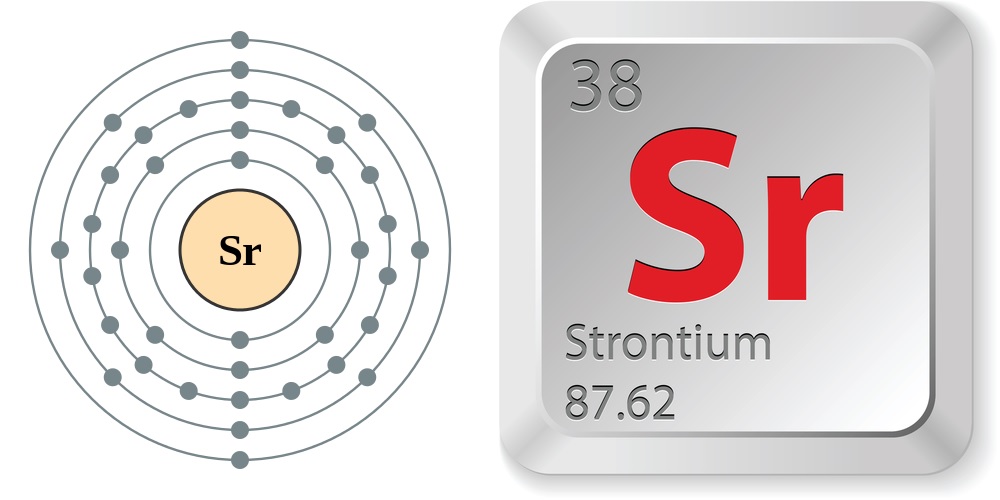
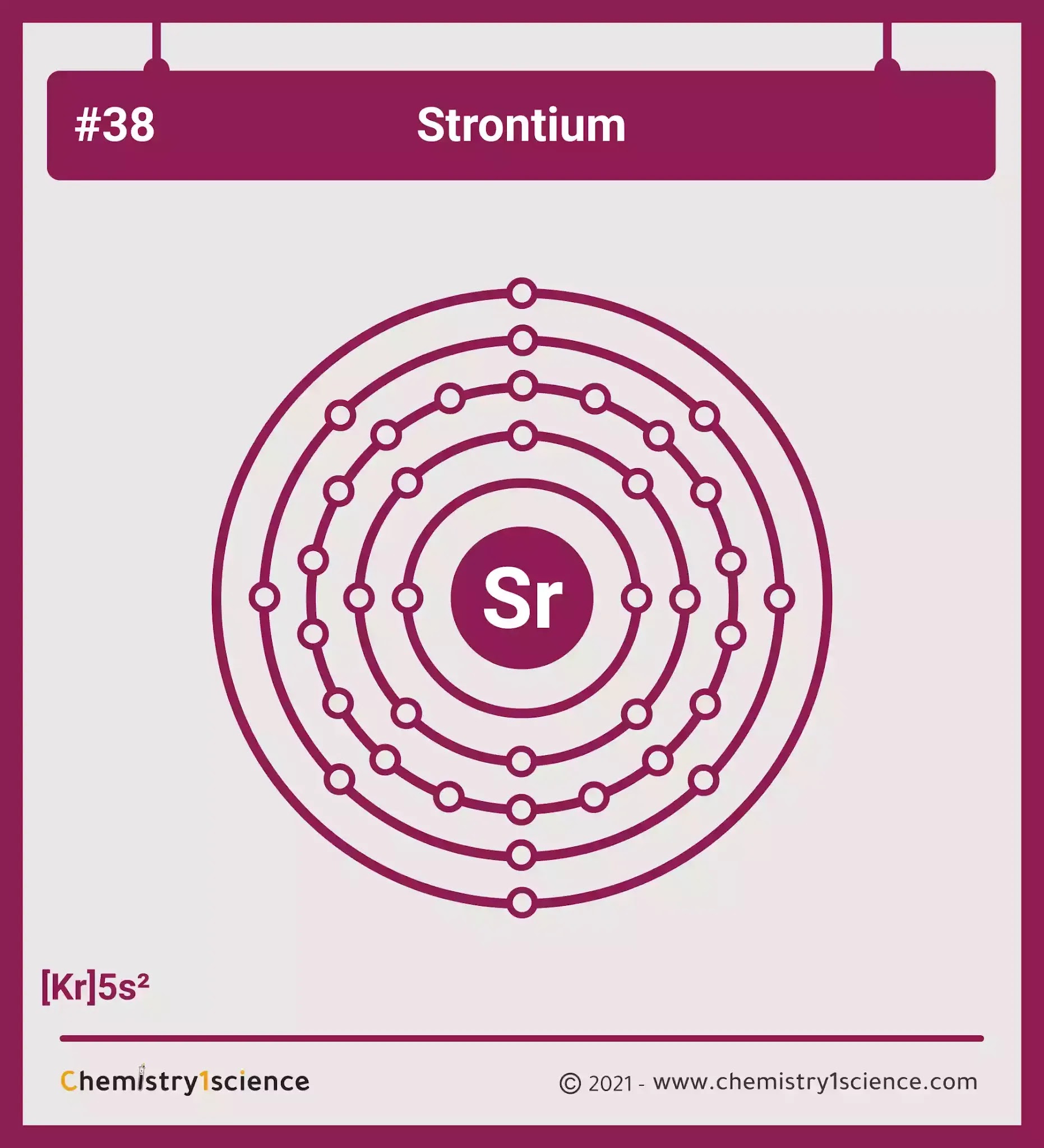
Strontium Electron Configuration Long Form
Strontium Electron Configuration Long Form - Web strontium may be obtained in the form of sticks by the contact cathode method of electrolysis, in which a cooled iron rod, acting as a cathode, just touches the surface of a fused mixture of potassium and strontium chlorides and. The chemical symbol for strontium is sr. The noble gas configuration of this element is [kr] 5s2, with [kr] representing the electron configuration of krypton. Number of neutrons (most common/stable nuclide): Web the electron configuration of strontium ion(sr 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. In order to write the sr electron. Electron configuration of erbium (er) [xe] 4f 12 6s 2. What is the abbreviated electronic configuration of strontium? 5s 2 electron dot model. Let me ask you a question. Hence, it lies in group 2. How to write the electron configuration for strontium (sr). In a neutral atom, an equal number of protons and electrons. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. The chemical symbol for strontium is sr. 1050 k (777 °c, 1431 °f) boiling point: This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of strontium atom has 2 electrons. Web we can write the electron configuration of strontium using four different methods: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. How to write the electron configuration for strontium (sr). The chemical symbol for strontium is sr. It is softer than calcium and decomposes water more vigorously. This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of strontium atom has 2 electrons. Web electron configuration of holmium (ho) [xe] 4f 11 6s 2. Web the main commercial process for strontium metal production is reduction of strontium oxide with aluminum. A slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). Web and the electron configuration of strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. 2, 8, 18, 30, 8, 2. In a neutral atom,. A slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the. Web strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure. It. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the. 2, 8, 18, 30, 8, 2. 5s 2 electron dot model. It occurs naturally only in compounds with other elements such as strontianite. Binding energies of common chemical states: Let me ask you a question. Hence, it lies in group 2. When liquid (at m.p.) 2.375 g/cm 3 : #4 from its orbital diagram. 2, 8, 18, 8, 2: How many shells does strontium have? #3 from its bohr model. 5s 2 electron dot model. The abbreviated electronic configuration of strontium is [kr] 5s2. In a neutral atom, an equal number of protons and electrons. Hence, it lies in group 2. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the. Naturally occurring strontium is a mixture of its four stable isotopes and they are found in the percentages shown: 2, 8,. First, find electrons of strontium atom. #3 from its bohr model. Web electron configuration of holmium (ho) [xe] 4f 11 6s 2. The chemical symbol for strontium is sr. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 11 6s 2. 2, 8, 18, 29, 8, 2. Web strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure. List of unique identifiers for strontium in various chemical registry databases. When liquid (at m.p.) 2.375 g/cm 3 : Web strontium is a group 2 element that does not occur as a free element due to its extreme reactivity with oxygen and water. The abbreviated electronic configuration of strontium is [kr] 5s2. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. Hence, it lies in group 2. 84 sr (0.6%), 86 sr (9.9%), 87. Full electron configuration of strontium: This electron configuration of strontium ion shows that the strontium ion(sr 2+) has acquired the electron configuration of krypton and it achieves a stable electron configuration. How many shells does strontium have? First, find electrons of strontium atom. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 11 6s 2. A slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). The commonly used long form of the periodic table is designed to emphasize electron configurations. Rb78 sr ii ground state 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 0 4s 2 4p 6 5s 2 s 1 / 2 ionization energy 88964.0 cm.Sr electronic configurationHow to write electronic configuration of
Indium(In) electron configuration and orbital diagram
How to write the electron configuration for Strontium (Sr). Electron
Symbol and electron diagram for Strontium illustration Stock Vector
Electron Shell Diagrams of the 118 Elements
How To Find an Valence Strontium Electron Configuration (Sr)
Strontium Periodic Table Protons Electrons 2022 Periodic Table Printable
Facts About Strontium Live Science
Electron Schematic Electron Configuration Of Strontium Excited State
Strontium Electron configuration Symbol Atomic Number Atomic
Related Post: