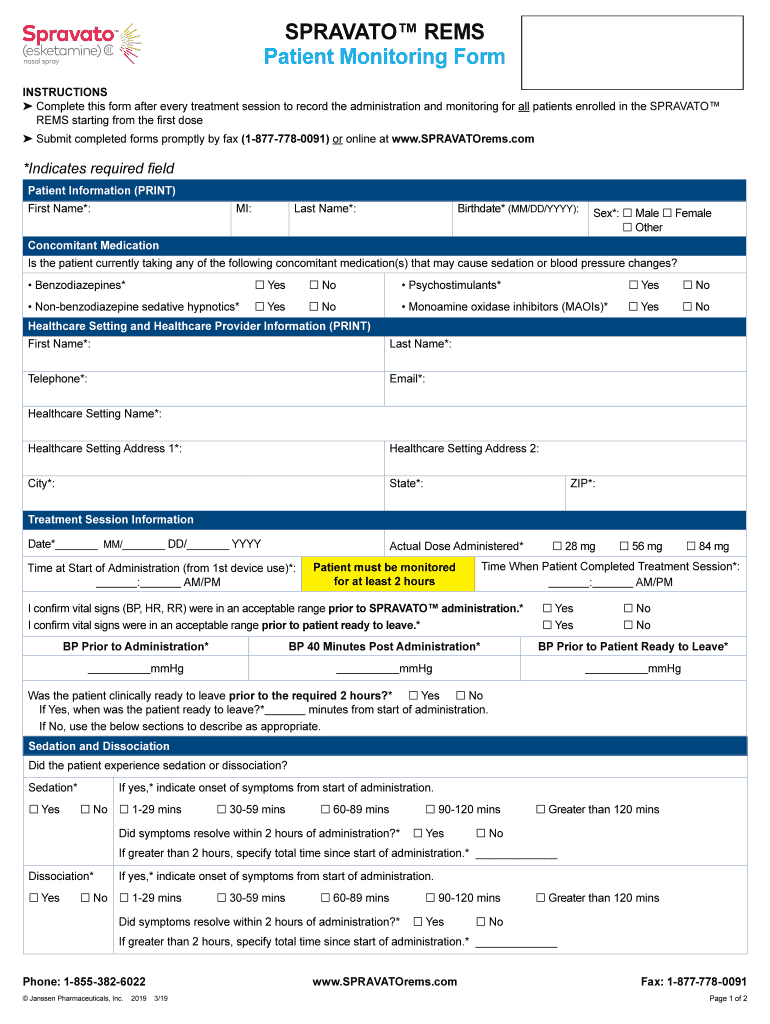
Spravato Rems Patient Monitoring Form
Spravato Rems Patient Monitoring Form - Web the rems program requires monitoring of the patient for ≥2 hours from the start of administration by a healthcare provider at each treatment session. Send the new spravato rems patient monitoring form in a digital form when you finish filling it out. Web spravato ® rems outpatient healthcare setting enrollments. Web download the patient discussion guide to learn more about the spravato® treatment experience and insurance, and help you discuss spravato® with your doctor. See full prescribing & safety info, including boxed. Web janssen scientific affairs, llc, a division of johnson & johnson's family of companies, is recruiting for a therapeutic area lead spravato (senior director) within. Outpatient healthcare settings must be certified in the spravato ® rems in order to prescribe product. Locate a certified spravato® treatment center. Ad visit the official site for product & safety information, including boxed warnings. Complete this form after every treatment session to record the administration and monitoring for all patients enrolled in the. Learn more about spravato® including safety information and talk to your doctor today. Complete this form after every treatment session to record the administration and monitoring for all patients enrolled in the. Spravato ® rems fact sheet. Web download the patient discussion guide to learn more about the spravato® treatment experience and insurance, and help you discuss spravato® with your. ≥65 6 fewer patients * spravato is not approved in pediatric patients. Ad visit the official site for product & safety information, including boxed warnings. Web spravato ® rems outpatient healthcare setting enrollments. Web the spravato rems requires esketamine to be dispensed and administered in medically supervised health care settings that are certified in the rems. Web spravato® is intended. Submit completed patient monitoring forms. Web spravato ® can intended for use only in a certified healthcare setting. Learn more about spravato® including safety information and talk to your doctor today. Web notable requirements of the zilbrysq rems include the following: Spravato ® rems fact sheet. Complete this form after every treatment session to record the administration and monitoring for all patients enrolled in the. Locate a certified spravato® treatment center. Web download clinical resources to learn more and educate your adult patients about how and when to use spravato®. Web download the patient discussion guide to learn more about the spravato® treatment experience and insurance,. Web the spravato rems requires esketamine to be dispensed and administered in medically supervised health care settings that are certified in the rems and agree to. Web notable requirements of the zilbrysq rems include the following: ≥65 6 fewer patients * spravato is not approved in pediatric patients. Web complete all required fields on this form after every treatment session. Locate a certified spravato® treatment center. Ad visit the official site for product & safety information, including boxed warnings. Web during spravato® treatment, submit the patient monitoring form and report all suspected adverse events to the spravato® rems *to get started, find more. Web for most commercial payers, include drug hcpcs coding (56 or 84 units of s0013) along with. Is your patient ready to begin treatment? The food and drug administration. Spravato ® rems fact sheet. Web complete all required fields on this form after every treatment session for all outpatients enrolled in the spravato® rems. Web during spravato® treatment, submit the patient monitoring form and report all suspected adverse events to the spravato® rems *to get started, find. Is your patient ready to begin treatment? Ad visit the official site for product & safety information, including boxed warnings. Locate a certified spravato® treatment center. Web janssen scientific affairs, llc, a division of johnson & johnson's family of companies, is recruiting for a therapeutic area lead spravato (senior director) within. ≥65 6 fewer patients * spravato is not approved. • prescribers must enroll in the rems. Web spravato ® can intended for use only in a certified healthcare setting. Web spravato® is intended for patient administration under the direct observation of a healthcare provider, and patients are required to be monitored by a healthcare provider. Web hit done and download the ecompleted template to your device. Locate a certified. Web spravato™ rems patient monitored submission full submittal of spravato® rems resigned monitoring form osmind is the single private practical ehr in this country. See full prescribing & safety info, including boxed. Is your patient ready to begin treatment? Locate a certified spravato® treatment center. Web download the patient discussion guide to learn more about the spravato® treatment experience and. Locate a certified spravato® treatment center. Web download the patient discussion guide to learn more about the spravato® treatment experience and insurance, and help you discuss spravato® with your doctor. Web for most commercial payers, include drug hcpcs coding (56 or 84 units of s0013) along with the relevant evaluation and monitoring codes. Web the spravato rems requires esketamine to be dispensed and administered in medically supervised health care settings that are certified in the rems and agree to. Web hit done and download the ecompleted template to your device. Web spravato ® can intended for use only in a certified healthcare setting. See full prescribing & safety info, including boxed. Web the spravato rems requires esketamine to be dispensed and administered in medically supervised health care settings that are certified in the rems. ≥65 6 fewer patients * spravato is not approved in pediatric patients. Web janssen scientific affairs, llc, a division of johnson & johnson's family of companies, is recruiting for a therapeutic area lead spravato (senior director) within. Locate a certified spravato® treatment center. Web during spravato® treatment, submit the patient monitoring form and report all suspected adverse events to the spravato® rems *to get started, find more. Web notable requirements of the zilbrysq rems include the following: Outpatient healthcare settings must be certified in the spravato ® rems in order to prescribe product. Spravato ® rems fact sheet. Ad visit the official site for product & safety information, including boxed warnings. Web during spravato® treatment, submit the patient monitoring form and report all suspected adverse events to the spravato® rems *to get started, find more. Submit completed patient monitoring forms. Send the new spravato rems patient monitoring form in a digital form when you finish filling it out. The food and drug administration.New Spravato FDA Approval Opens The Door to New Patients, Says Sandhya
J&J's ketamine depression drug Spravato spurned by England's
Spravato Patient Monitoring Form Fill Out and Sign Printable PDF
U.S. FDA อนุมัติยา Spravato Nasal Spray สำหรับผู้ป่วยโรคซึมเศร้า อยู่
The FDA approved Spravato fastacting treatment of depression https
EsKetamine Johnson City SPRAVATO™ FDA Approved Treatment
SPRAVATO™ (Esketamine) New FDA approved treatment for Treatment
FDA approves Spravato, a fastacting antidepressant nasal spray TMC News
Spravato™ nasal spray
Stung by critics, J&J says antidepressant Spravato saves money PMLiVE
Related Post: