Reaction Of Hydrogen And Nitrogen To Form Ammonia
Reaction Of Hydrogen And Nitrogen To Form Ammonia - What is the theoretical yield of nh3 in grams?. When the concentration of nitrogen is increased, the rate of production of ammonia will a glass of milk is initially at room. Web 86% (35 ratings) transcribed image text: Web the reaction between nitrogen and hydrogen to form ammonia is carried out in a flask containing 30.5 g of n2 and 8.65 g of h2. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of ammonia. Hydrogen was preheated to 300°c from vapors leaving the ammonia synthesis reactor. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. Hydrogen gas and nitrogen gas react to form ammonia gas. Hence, if nitrogen and hydrogen. Web liquid hydrogen carriers will soon play a significant role in transporting energy. 3 h 2 ( g) hydrogen + n 2. Nitrogen and hydrogen react to form ammonia according to the following balanced. Web the pressurised gases are pumped into a tank containing beds of iron catalyst at about 450°c. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. Web nitrogen and. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of ammonia. Hydrogen and nitrogen react to form ammonia. Web nitrogen and hydrogen combine to form ammonia in the haber process. Web the pressurised gases are pumped into a tank containing beds of iron catalyst at about 450°c. Furthermore, four. What is the theoretical yield of nh3 in grams?. Suppose you have 13.0 mol n2 of and 9.0 h2 of in a reactor. Nitrogen and hydrogen react to form ammonia according to the following balanced. Web liquid hydrogen carriers will soon play a significant role in transporting energy. Hydrogen and nitrogen react to form ammonia. N2 ( g)+3h2 ( g)→2nh3 ( g) if all the n2 and h2 are consumed, what volume of nh3, at the. The synthesis of ammonia equation is shown as the following:. Web 86% (35 ratings) transcribed image text: H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g) ammonia. The standard enthalpy change (δh°). Web nitrogen and hydrogen combine to form ammonia in the haber process. Web the formation of ammonia from nitrogen and hydrogen is mildly exothermic: Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. Web. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of ammonia. Nitrogen and hydrogen react to form ammonia according to the following balanced. Web liquid hydrogen carriers will soon play a significant role in transporting energy. Since the reaction is exothermic, the equilibrium of the reaction shifts at lower. Suppose you have 13.0 mol n2 of and 9.0 h2 of in a reactor. Web the reaction between nitrogen and hydrogen to form ammonia is carried out in a flask containing 30.5 g of n2 and 8.65 g of h2. The chemical equation for this reaction is shown. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with. About 46 kj/mol for anhydrous ammonia, ~80 kj/mol for aqueous. Web liquid hydrogen carriers will soon play a significant role in transporting energy. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g) ammonia. Hence, if nitrogen and hydrogen. Web the reaction between nitrogen and hydrogen to form ammonia is carried out in a. In these conditions, some of the hydrogen and nitrogen will react to form. When the concentration of nitrogen is increased, the rate of production of ammonia will a glass of milk is initially at room. Since the reaction is exothermic, the equilibrium of the reaction shifts at lower temperatures to the ammonia side. Web nitrogen and hydrogen react to form. About 46 kj/mol for anhydrous ammonia, ~80 kj/mol for aqueous. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of ammonia. Web nitrogen and hydrogen combine to form ammonia in the haber process. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia. Web what amount (in moles)of hydrogen will produce 44.0 mol of ammonia? Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of ammonia. Web nitrogen and hydrogen combine to form ammonia in the haber process. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. If 4.0 moles of h2 with 2.0 moles of n2 are reacted, how many. The standard enthalpy change (δh°) for a reaction can be. N2 (g) +3h2 (g)→2nh3 (g) imagine 119.mmol of nh3 are added to an empty flask, and then answer the following. Hydrogen was preheated to 300°c from vapors leaving the ammonia synthesis reactor. Since the reaction is exothermic, the equilibrium of the reaction shifts at lower temperatures to the ammonia side. Web the reaction between nitrogen and hydrogen to form ammonia is carried out in a flask containing 30.5 g of n2 and 8.65 g of h2. The key factors that are considered when assessing the applicability of. Hydrogen and nitrogen react to form ammonia. Web liquid hydrogen carriers will soon play a significant role in transporting energy. Web nitrogen and hydrogen react to form ammonia. Web ammonia is consistently among the top five chemicals produced in the united states. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. Nitrogen and hydrogen react to form ammonia according to the following balanced. Nitrogengas and hydrogengas react to form ammoniagas. Web 86% (35 ratings) transcribed image text: Web nitrogen and hydrogen react to form ammonia, like this:D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
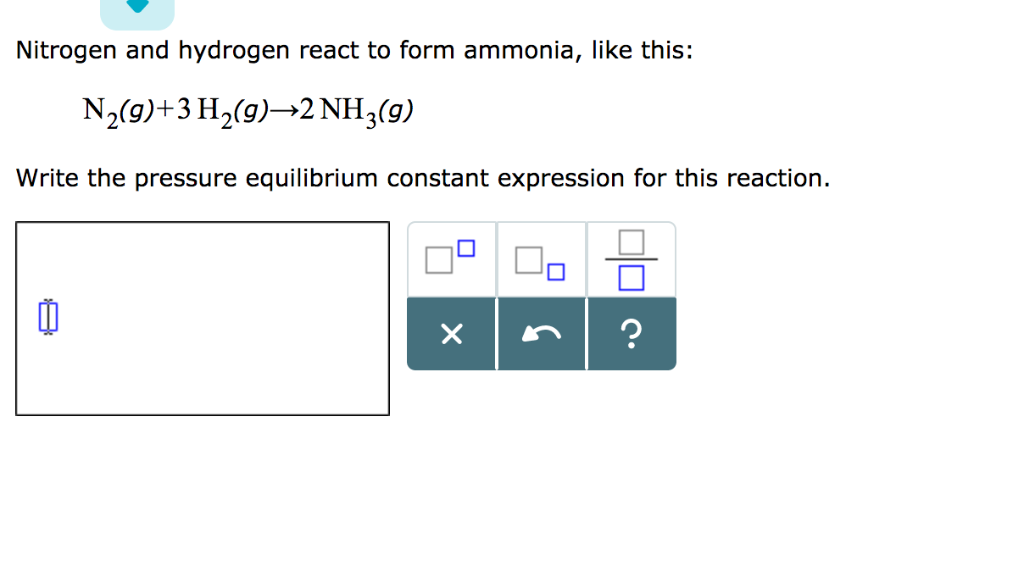
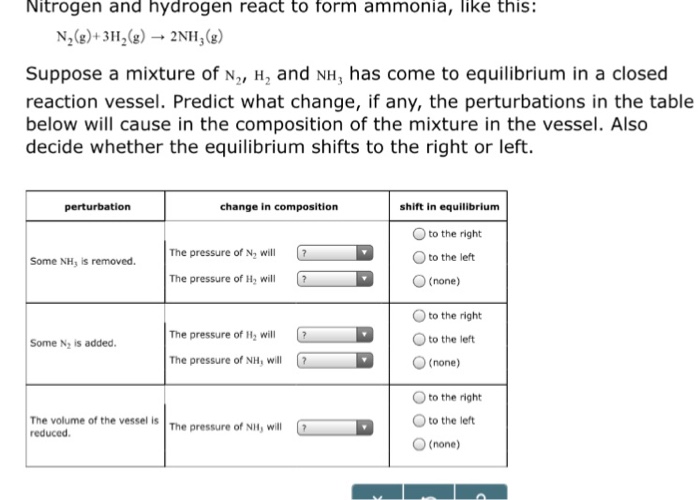
Solved Nitrogen and hydrogen react to form ammonia, like
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
Solved Nitrogen and hydrogen react to form ammonia, like
PPT Consider the reaction for the formation of ammonia from nitrogen
PPT Balancing Equations ANSWER KEY PowerPoint Presentation, free
Solved Nitrogen and hydrogen react to form ammonia, like
Solved Nitrogen And Hydrogen Combine To Form Ammonia In T...
Hydrogen reacts with nitrogen to produce ammonia according to this
Solved Nitrogen and hydrogen react to produce ammonia
Related Post: