Phs Human Subjects And Clinical Trials Information Form
Phs Human Subjects And Clinical Trials Information Form - If yes to human subjects 78 add a record for each proposed human subject study by. Ad put the experience of our mobile research nurses to work for your trial. Web the phs human subjects and clinical trials information form is used to collect information on: The form consolidates into a single. Learn why we're a preferred mobile research provider for decentralized clinical trials. Skip the rest of the phs human subjects and clinical trials information. Supports versions2 & 3 fornih formseriesf. Web the new phs human subject and clinical trial information form will flag trials, helping us to achieve a number of goals. The following items are taken from the. 03/31/2020 please complete the human subjects section of the. 03/31/2020 please complete the human subjects section of the. Ad signnow.com has been visited by 100k+ users in the past month Web phs human subjects and clinical trials information created date: Web skip the rest of the phs human subjects and clinical trials information form. Web the phs human subjects and clinical trials information form allows you to add study. Ad pdffiller.com has been visited by 1m+ users in the past month Web thephs humansubjects& clinicaltrialinformation form. Supports versions2 & 3 fornih formseriesf. Web the new phs human subject and clinical trial information form will flag trials, helping us to achieve a number of goals. Skip the rest of the phs human subjects and clinical trials information. Inclusion of women, minorities, and. Web about the phs human subject and clinical trial information form. Web if yes, provide an explanation of why the application does not involve human subjects research. Web the phs human subjects and clinical trials information form is used to collect information on human subjects research, clinical research, and/or clinical trials,. Web skip the rest. Web about the phs human subject and clinical trial information form. Ad signnow.com has been visited by 100k+ users in the past month The form consolidates into a single. Web the phs human subjects and clinical trials information form is used to collect information on human subjects research, clinical research, and/or clinical trials,. Web the phs human subjects and clinical. Web if yes, provide an explanation of why the application does not involve human subjects research. Web thephs humansubjects& clinicaltrialinformation form. Web the phs human subjects and clinical trials information form is used to collect information on human subjects research, clinical research, and/or clinical trials,. Inclusion of women, minorities, and. Nih issues the *new* phs human subjects and clinical trials. Nih issues the *new* phs human subjects and clinical trials information form (grants.gov. Ad signnow.com has been visited by 100k+ users in the past month The form consolidates into a single. Learn why we're a preferred mobile research provider for decentralized clinical trials. Web the phs human subjects and clinical trials information form is used to collect information on human. Web the phs human subjects and clinical trials information form is used to collect information on: 03/31/2020 please complete the human subjects section of the. Learn why we're a preferred mobile research provider for decentralized clinical trials. Ad put the experience of our mobile research nurses to work for your trial. Web if yes, provide an explanation of why the. Inclusion of women, minorities, and. Web the new phs human subject and clinical trial information form will flag trials, helping us to achieve a number of goals. Web phs human subjects and clinical trials information created date: Web the phs human subjects and clinical trials information form will capture detailed study information, including eligibility criteria; Human subjects research clinical research. The form consolidates into a single. Supports versions2 & 3 fornih formseriesf. Web phs human subjects and clinical trials information created date: Web the phs human subjects and clinical trials information form will capture detailed study information, including eligibility criteria; Web the phs human subjects and clinical trials information form is used to collect information on: Ad signnow.com has been visited by 100k+ users in the past month Web the phs human subjects and clinical trials information form allows you to add study record (s) and/or delayed onset study (ies), as applicable. Web the phs human subjects and clinical trials information form will capture detailed study information, including eligibility criteria; Learn why we're a preferred mobile. Web the phs human subjects and clinical trials information form is used to collect information on human subjects research, clinical research, and/or clinical trials,. Web the phs human subjects and clinical trials information form will capture detailed study information, including eligibility criteria; Web the phs human subjects and clinical trials information form will capture detailed study information, including eligibility criteria; Web about the phs human subject and clinical trial information form. The form consolidates into a single. The following items are taken from the. If yes to human subjects 78 add a record for each proposed human subject study by. Web phs human subjects and clinical trials information created date: Inclusion of women, minorities, and. Web skip the rest of the phs human subjects and clinical trials information form. Web thephs humansubjects& clinicaltrialinformation form. Learn why we're a preferred mobile research provider for decentralized clinical trials. Web phs human subjects and clinical trials information. Supports versions2 & 3 fornih formseriesf. Web if yes, provide an explanation of why the application does not involve human subjects research. Human subjects research clinical research and/or clinical. Web the phs human subjects and clinical trials information form allows you to add study record (s) and/or delayed onset study (ies), as applicable. Ad signnow.com has been visited by 100k+ users in the past month Ad put the experience of our mobile research nurses to work for your trial. Web the new phs human subject and clinical trial information form will flag trials, helping us to achieve a number of goals.G.500 PHS Human Subjects and Clinical Trials Information
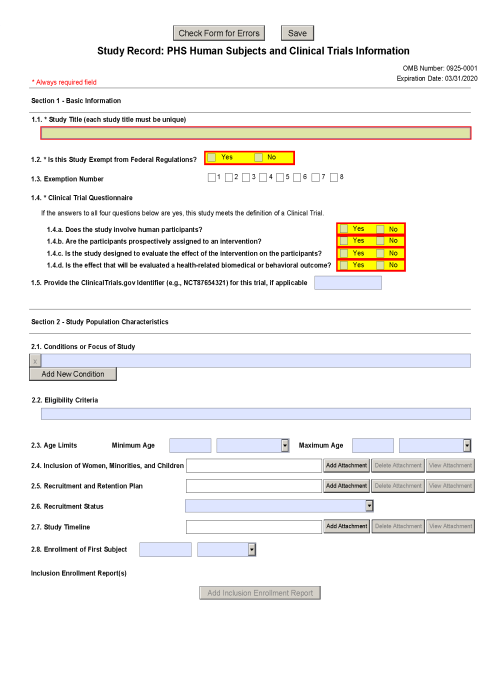
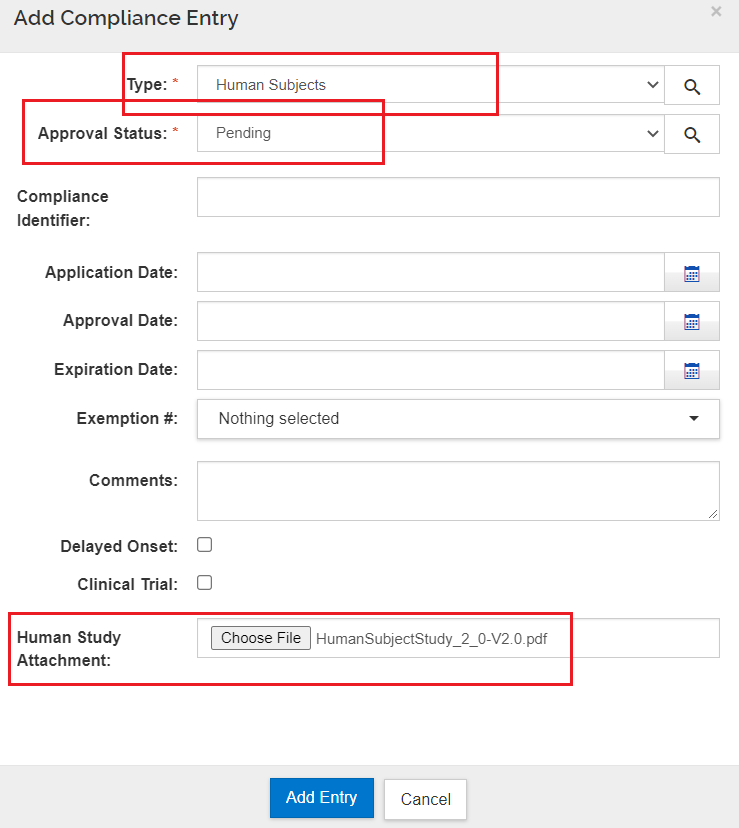
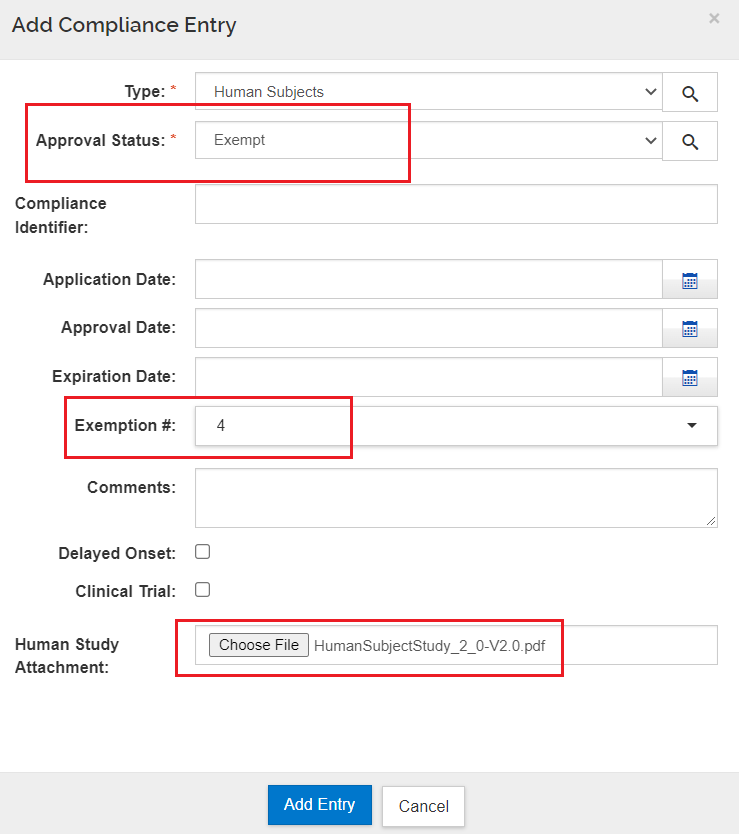
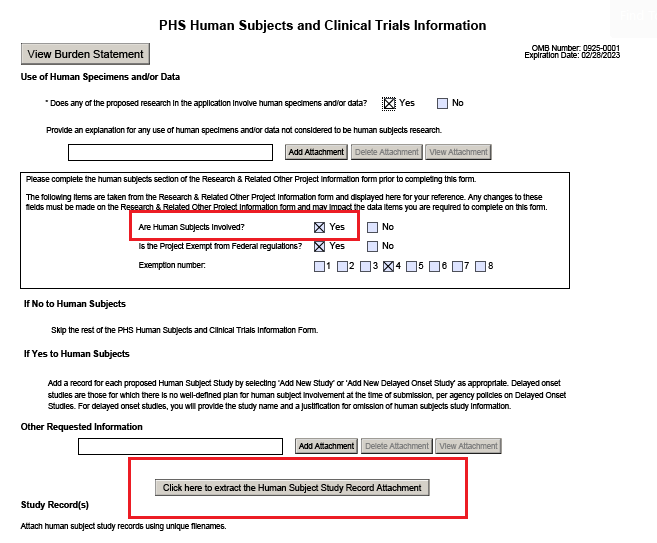
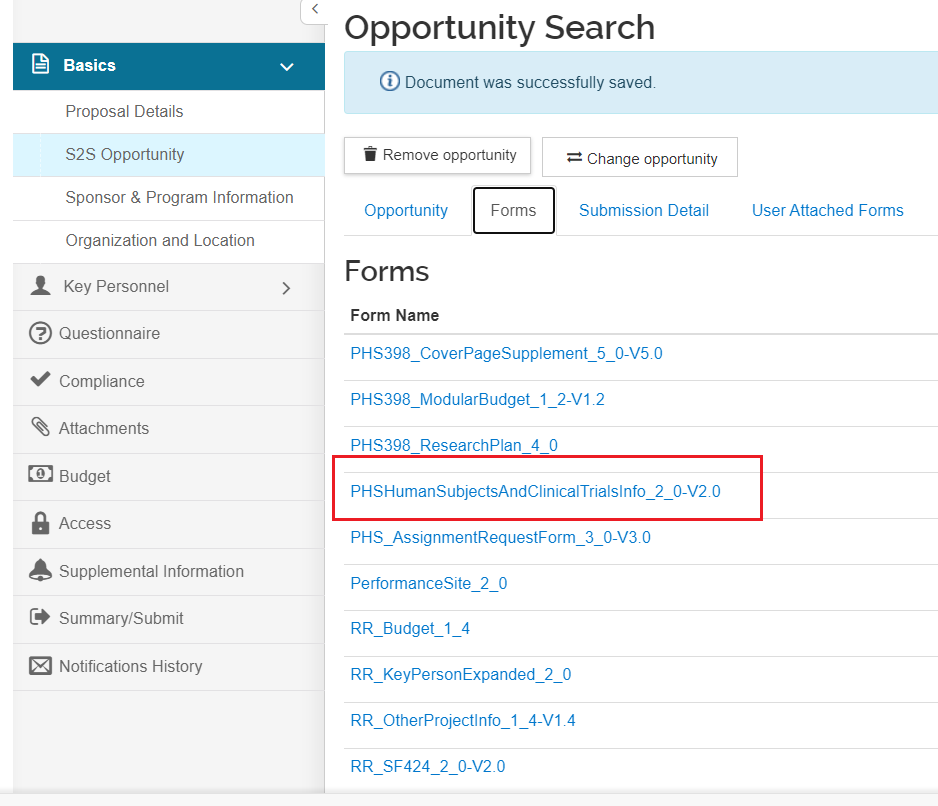
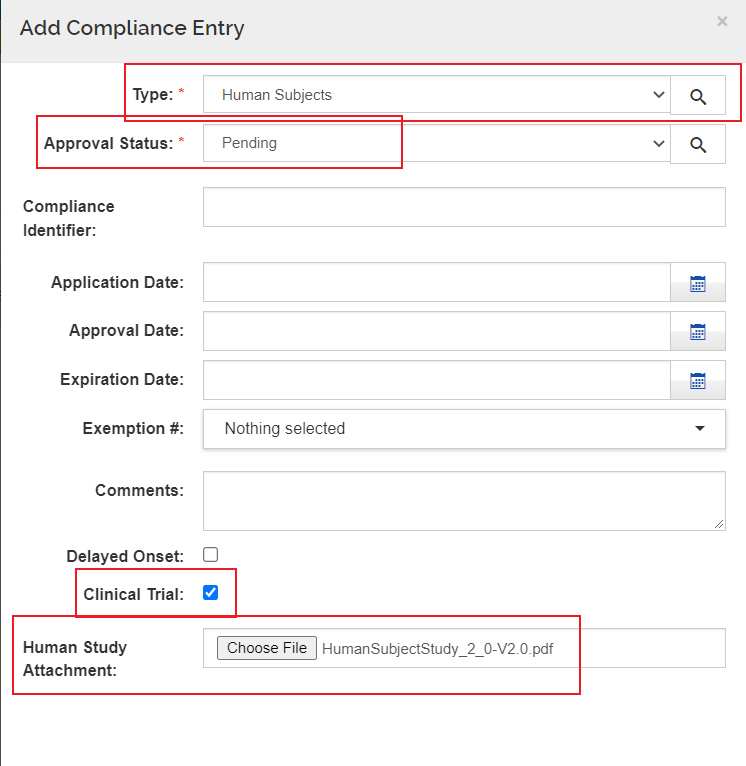
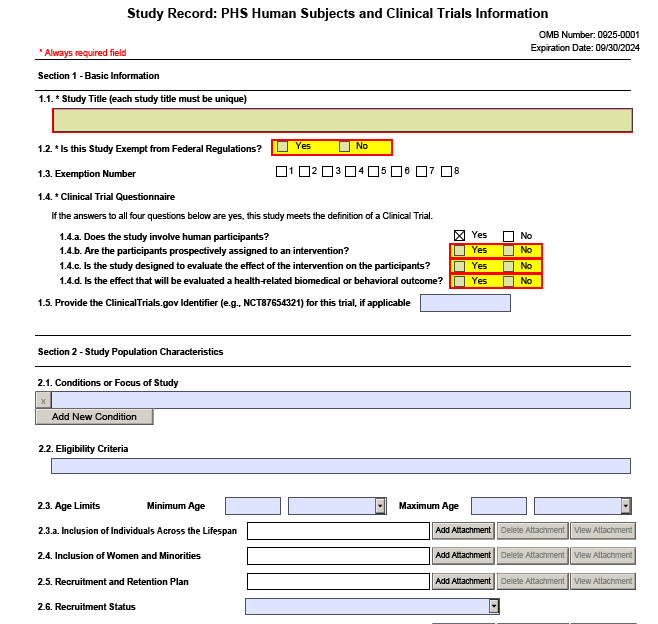
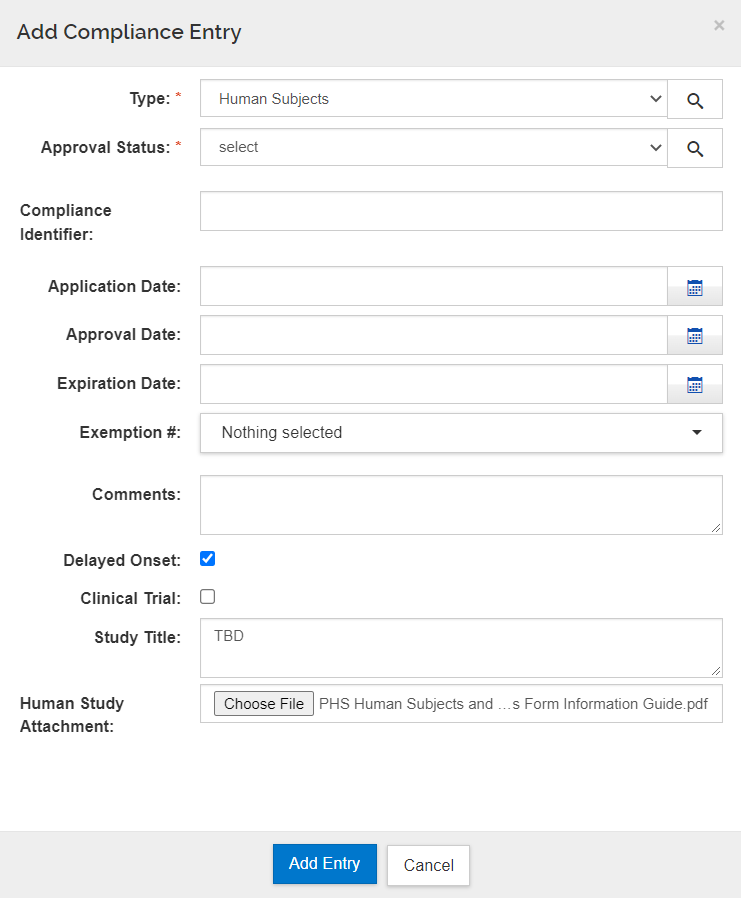
Form Screenshots
PHS Human Subject and Clinical Trials Form Information Guide MSU
PHS Human Subject and Clinical Trials Form Information Guide MSU
PHS Human Subjects and Clinical Trials Information Form Walkthrough
PHS Human Subject and Clinical Trials Form Information Guide MSU
PHS Human Subject and Clinical Trials Form Information Guide MSU
PHS Human Subject and Clinical Trials Form Information Guide MSU
PHS Human Subject and Clinical Trials Form Information Guide MSU
PHS Human Subject and Clinical Trials Form Information Guide MSU
Related Post: