Phosphorus Electron Configuration Long Form
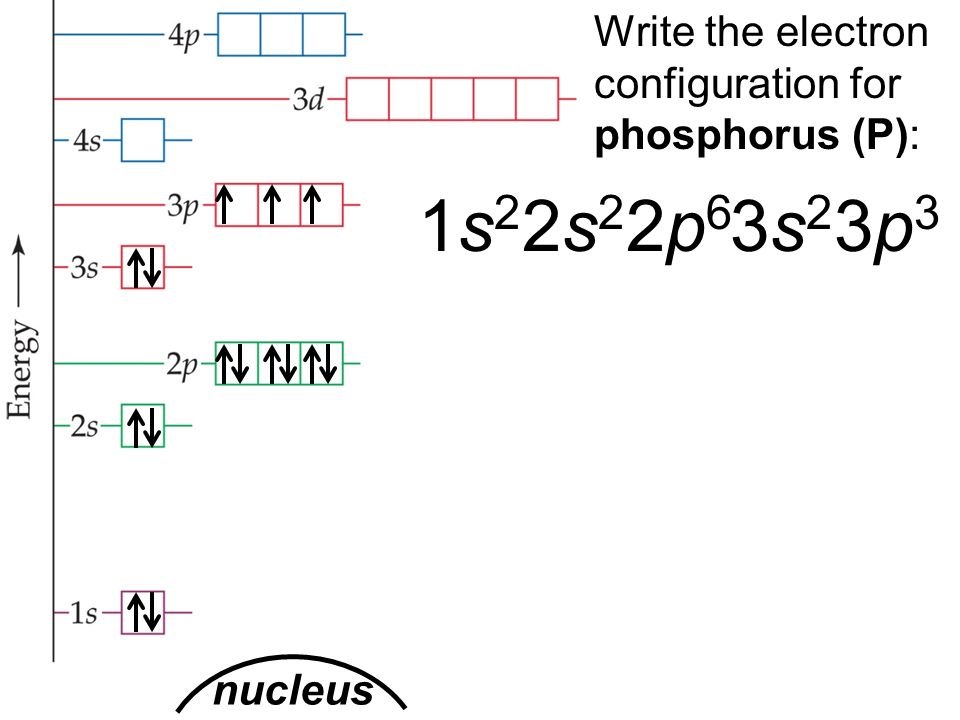
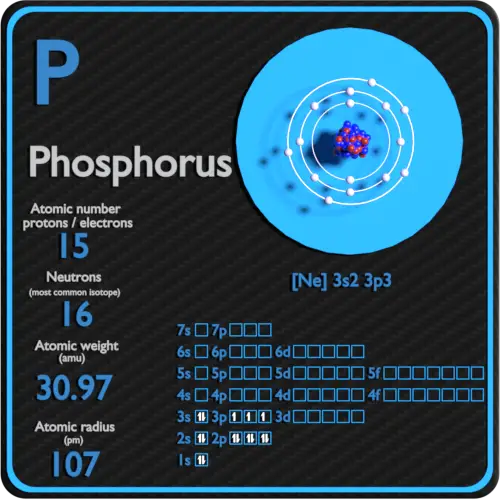
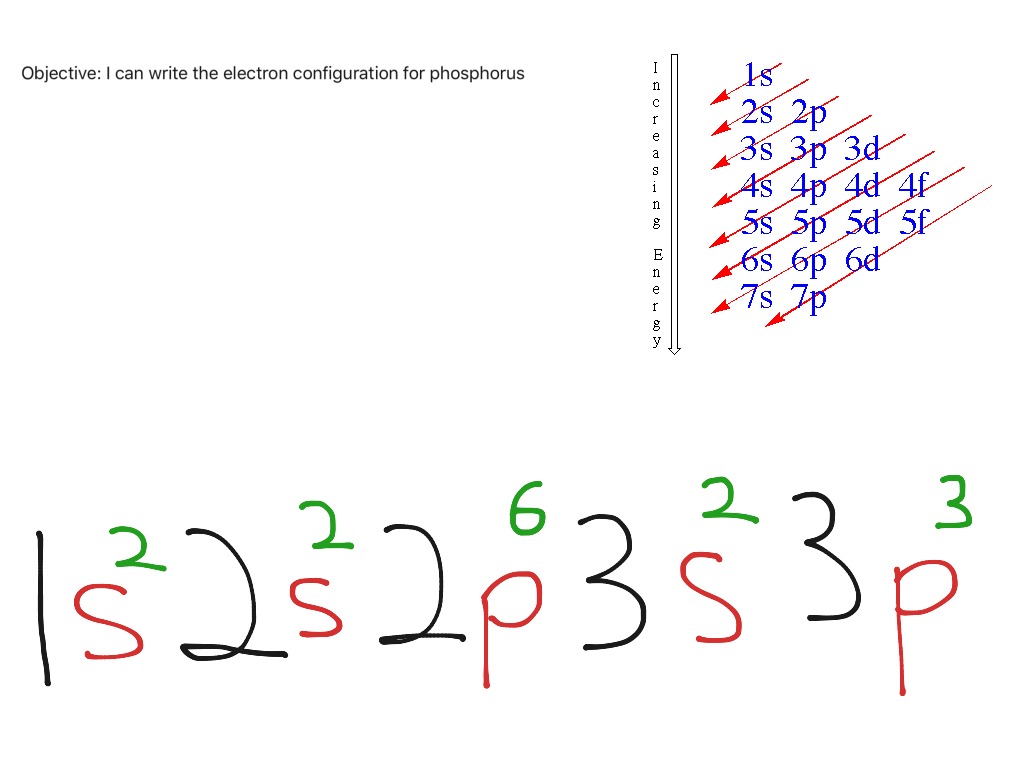
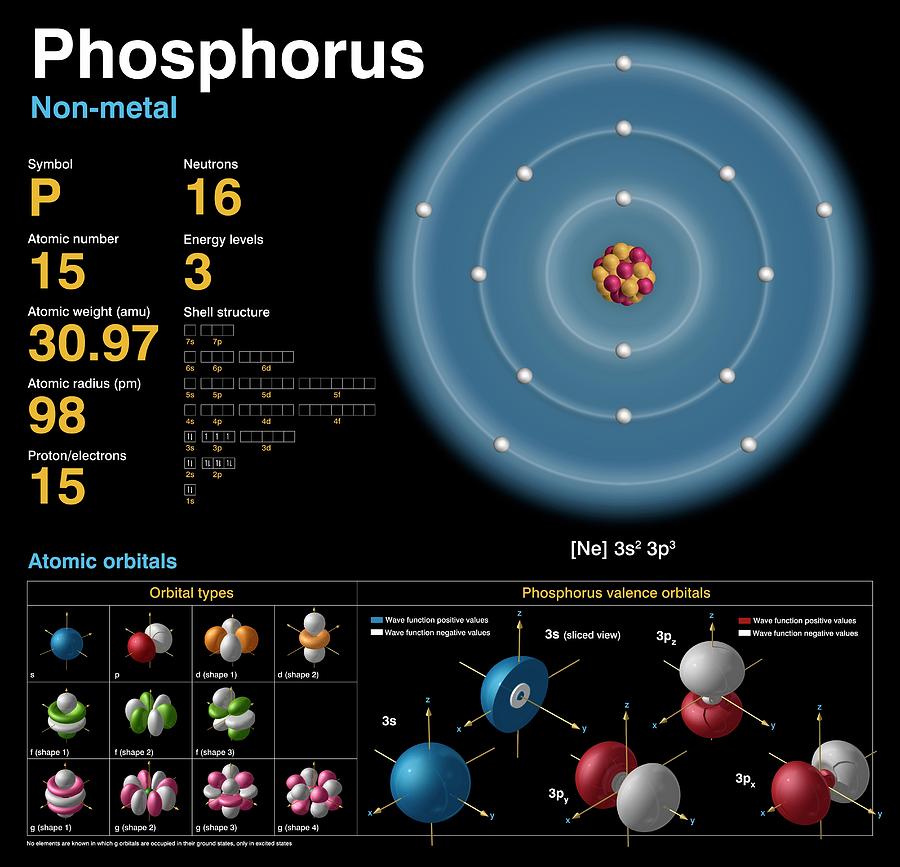
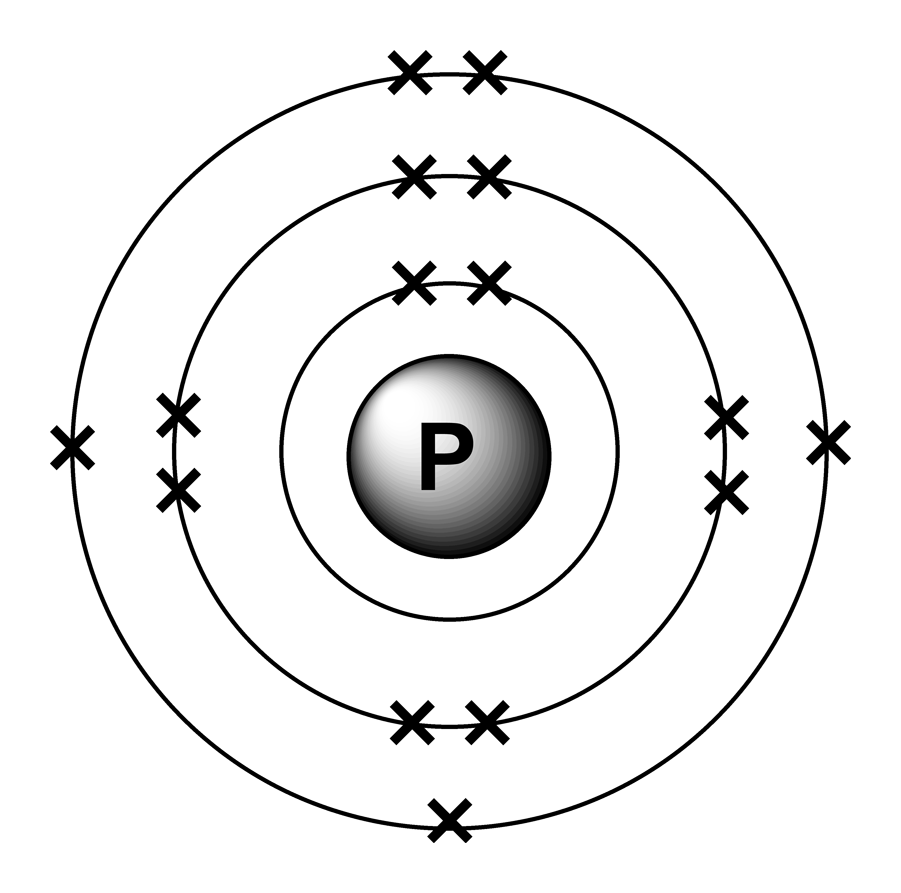
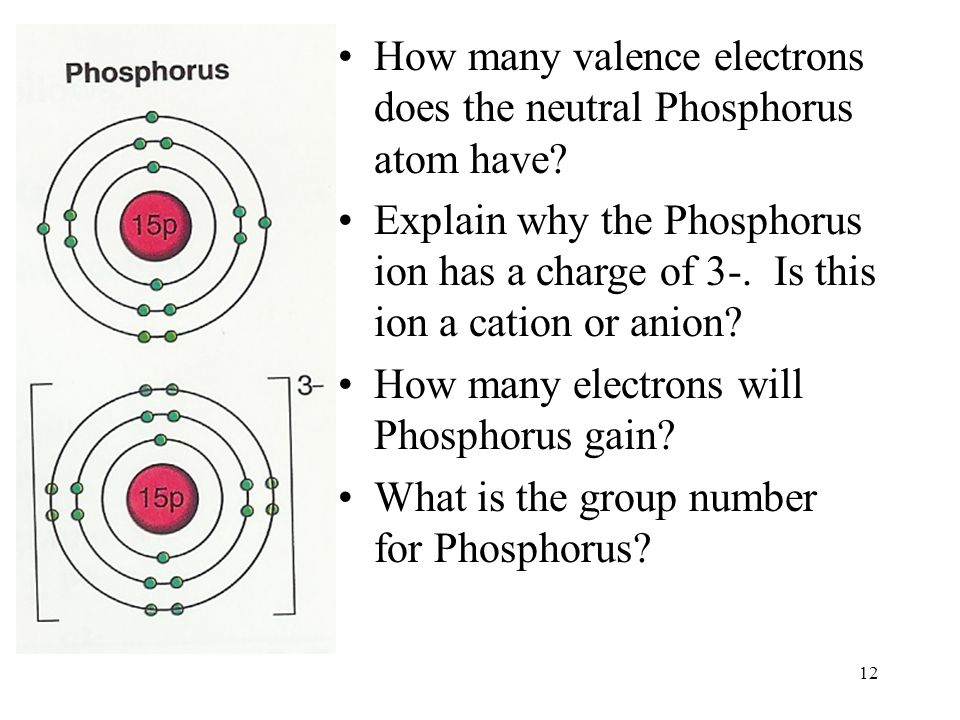
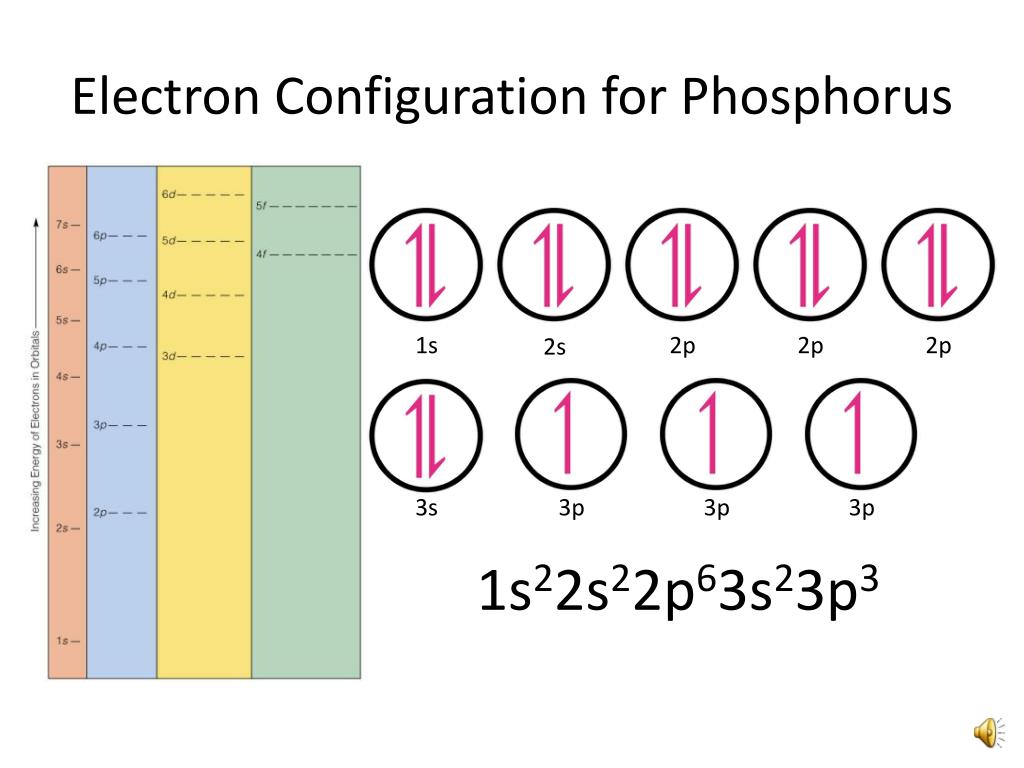
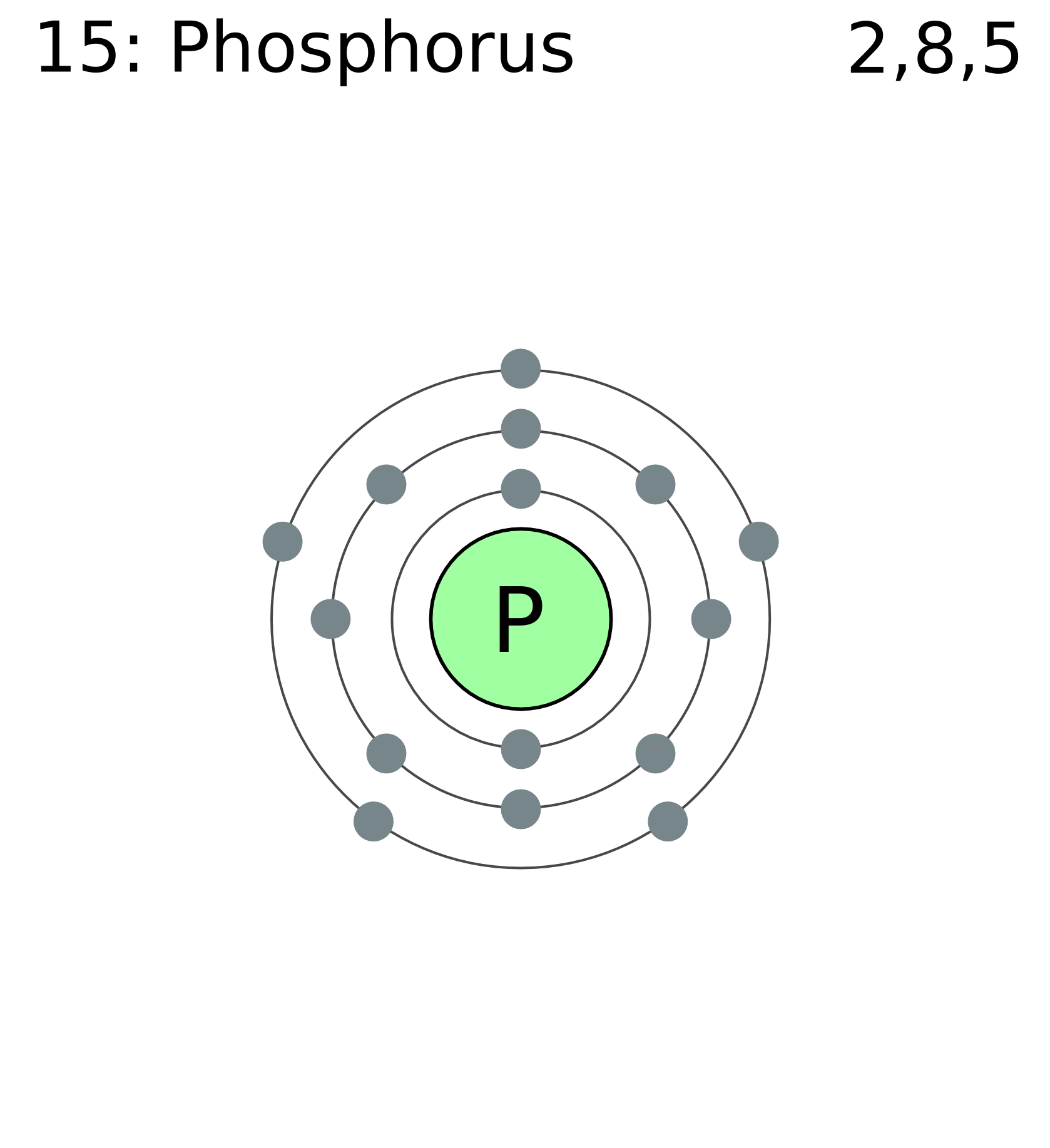
Phosphorus Electron Configuration Long Form - Since the 3s if now full we'll move to the 3p where we'll place the. We'll put six in the 2p orbital and then put the next two electrons in the 3s. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. 1.5k views 1 year ago organic chemistry course. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. A single phosphorus atom has 15 protons and 15. 5, 4, 3, 2, 1, −1,. Web the p orbital can hold up to six electrons. Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. Let's find the phosphorus electron configuration! Web the p orbital can hold up to six electrons. Web how to write/find/do the phosphorus (p) ground state/long form/short form/longhand/short hand/abbreviated/condensed/noble gas notation electron configuratio. We'll put six in the 2p orbital and then put the next two electrons in the 3s. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. Web electron configuration the arrangements. Let's find the phosphorus electron configuration! Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Describe the general characteristics of the first (lowest. A single phosphorus atom has 15 protons and 15. Web this representation is shown in the form of some equation that we generally refer to as the electron configuration. Describe the general characteristics of the first (lowest. Web the p orbital can hold up to six electrons. (a) b3+ (b) o (c) cl3+ (d) ca2+ (e) ti. Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. 2 electrons in the 3 s. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. Web the electrons that identify the phosphorous element are modified or structured according to the model of the electron shells, their configuration is as follows: Phosphorus has 15 electrons distributed across its energy levels and orbitals. The electron configuration of this chemical. Web the electron configuration of phosphorus. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web electron configuration 3s 2 3p 3: Web the electron configuration of phosphorus is 1s^2 2s^2 2p^6 3s^2 3p^3. (a) b3+ (b) o (c) cl3+ (d) ca2+ (e) ti. 101k views 9 years ago. Web this representation is shown in the form of some equation that we generally refer to as the electron configuration. The electron configuration of this chemical. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the. 5, 4,. Web how to write/find/do the phosphorus (p) ground state/long form/short form/longhand/short hand/abbreviated/condensed/noble gas notation electron configuratio. Web write the electron configurations for the following atoms or ions: 280.5 (white phosphorus) electron configuration: Web this representation is shown in the form of some equation that we generally refer to as the electron configuration. (a) b3+ (b) o (c) cl3+ (d) ca2+. 280.5 (white phosphorus) electron configuration: Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web electron configuration 3s 2 3p 3: A single phosphorus atom has 15 protons and 15. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Phosphorus has 15 electrons distributed across its energy levels and orbitals. 1.5k views 1 year ago organic chemistry course. 280.5 (white phosphorus) electron configuration: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. 2 electrons in the 3 s subshell and. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. Web electron configuration the arrangements. Web the electrons that identify the phosphorous element are modified or structured according to the model of the electron shells, their configuration is as follows: Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Describe the general characteristics of the first (lowest. 1.5k views 1 year ago organic chemistry course. Let's find the phosphorus electron configuration! Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. Web the electron configuration of phosphorus is 1s^2 2s^2 2p^6 3s^2 3p^3. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. We'll put six in the 2p orbital and then put the next two electrons in the 3s. 5, 4, 3, 2, 1, −1,. (a) b3+ (b) o (c) cl3+ (d) ca2+ (e) ti. 2 electrons in the 3 s subshell and. Web the p orbital can hold up to six electrons. Web 44.15 (white phosphorus) atomic weight: Web write the electron configurations for the following atoms or ions: Web electron configuration 3s 2 3p 3: 280.5 (white phosphorus) electron configuration: Web thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Phosphorus has 15 electrons distributed across its energy levels and orbitals.Phosphorus Electron Configuration (P) with Orbital Diagram
Phosphorus Protons Neutrons Electrons Electron Configuration
Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
Electron configuration example for phosphorus Science, Chemistry
Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
Phosphorus Electron Configuration YouTube
Electron configurations
Phosphorus Periodic Table Protons Neutrons Electrons Periodic Table
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
Phosphorus Electron Configuration (P) with Orbital Diagram
Related Post: