Patient Status Form Clozapine Rems
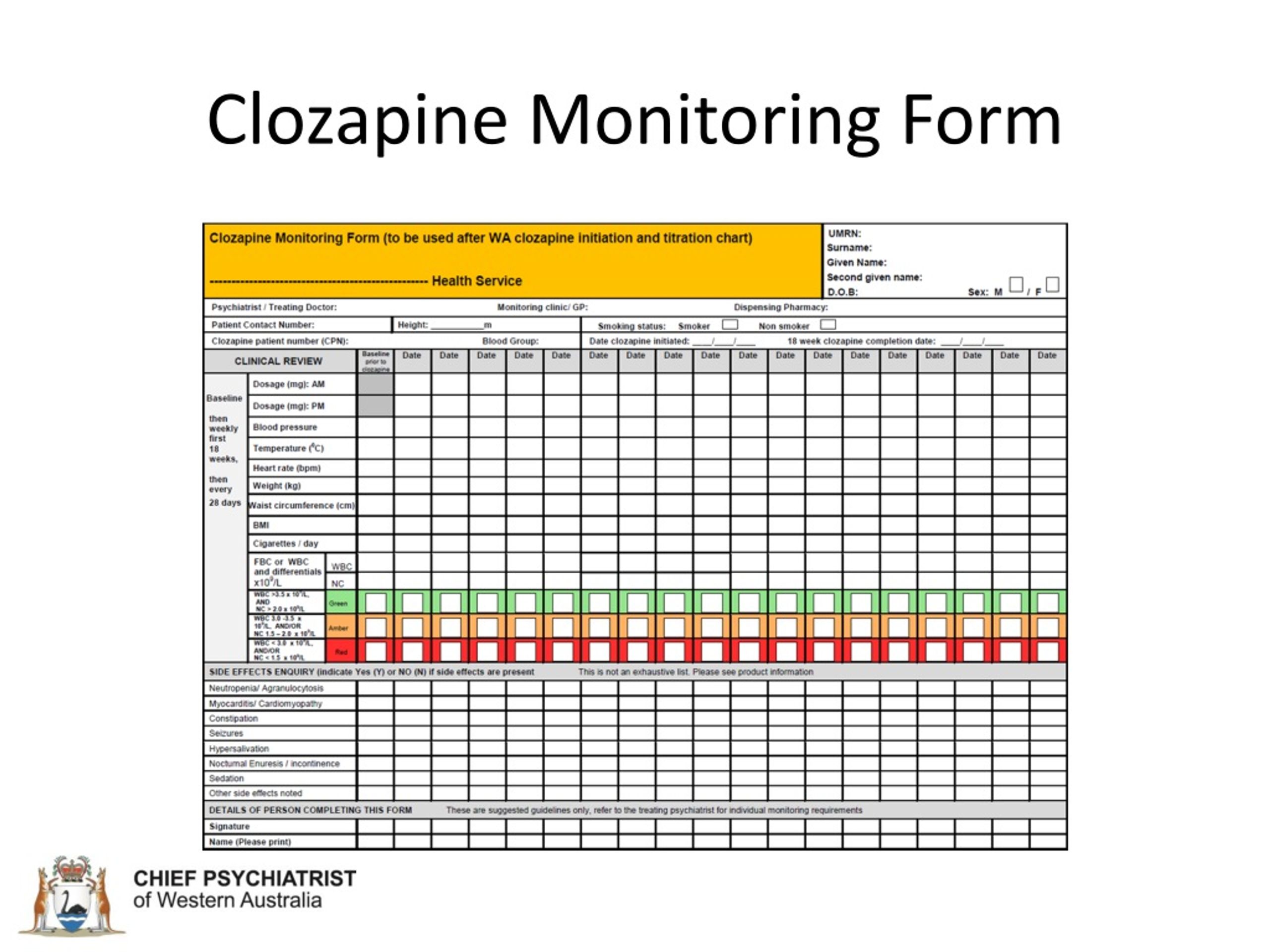
Patient Status Form Clozapine Rems - Web the clozapine rems resulted from the fda amendments act of 2007 which gave the fda power to require rems programs. Patient monitoring must still continue per the. A new patient status form (psf) will be used to document absolute. Web rems include a risk mitigation goal, and are comprised of information communicated to and/or required activities to be undertaken by one or more participants (i.e., healthcare. To certify as a prescriber: Web we encourage pharmacists and prescribers to continue working with the clozapine rems to complete certification and prescribers should continue to enroll. Web beginning on november 15, the clozapine rems requires a new patient status form to document monitoring for all outpatients. Web • must verify and document each clozapine patient’s ancs to the clozapine rems monthly, by submitting the patient status form. It's simple to start saving today at the pharmacy. Web the new patient status is used to document monitoring of the anc. Web we would like to show you a description here but the site won’t allow us. Web rems include a risk mitigation goal, and are comprised of information communicated to and/or required activities to be undertaken by one or more participants (i.e., healthcare. Web beginning on november 15, the clozapine rems requires a new patient status form to document monitoring. A new patient status form (psf) will be used to document absolute. To receive treatment, a patient must be enrolled in the clozapine rems program by a certified doctor. This form must be submitted monthly. Web clozapine rems is intended to maximize the benefits of the drug and minimize risk. Currently, there is reluctance amongst some clinicians to prescribe. Ad print your clozapine coupon instantly or bring it to the pharmacy on your phone. The form is used to document the monitoring frequency, anc values, adverse events related to. Web rems include a risk mitigation goal, and are comprised of information communicated to and/or required activities to be undertaken by one or more participants (i.e., healthcare. Web the new. This form must be submitted monthly. Although such orders may be processed with a manual pharmacy review of the stop date(s) in the pharmacy. Web • must verify and document each clozapine patient’s ancs to the clozapine rems monthly, by submitting the patient status form. Web new patient status form to document monitoring for all outpatients. Healthcare providers must be. Web the clozapine rems resulted from the fda amendments act of 2007 which gave the fda power to require rems programs. This form must be submitted monthly. Web beginning on november 15, the clozapine rems requires a new patient status form to document monitoring for all outpatients. Healthcare providers must be certified in the clozapine rems to prescribe for outpatient. No commitment or fees to use goodrx. Web rems include a risk mitigation goal, and are comprised of information communicated to and/or required activities to be undertaken by one or more participants (i.e., healthcare. The form is used to document the monitoring frequency, anc values, adverse events related to clozapine. Web we encourage pharmacists and prescribers to continue working with. To certify as a prescriber: 1 clozapine is a second. No commitment or fees to use goodrx. Web clozapine rems is intended to maximize the benefits of the drug and minimize risk. Web rems include a risk mitigation goal, and are comprised of information communicated to and/or required activities to be undertaken by one or more participants (i.e., healthcare. Ad print your clozapine coupon instantly or bring it to the pharmacy on your phone. Page 3 of 3 information about the new. Web • must verify and document each clozapine patient’s ancs to the clozapine rems monthly, by submitting the patient status form. A new patient status form (psf) will be used to document absolute. This form must be. Web patient’s clozapine rems monitoring frequency. Web rems include a risk mitigation goal, and are comprised of information communicated to and/or required activities to be undertaken by one or more participants (i.e., healthcare. Web clozapine rems is intended to maximize the benefits of the drug and minimize risk. Web we encourage pharmacists and prescribers to continue working with the clozapine. Web delaying patients getting clozapine is going to limit the extent of response. Web the new patient status form is used to document monitoring of the anc. Page 3 of 3 information about the new. Web a new patient status form will document absolute neutrophil count (anc) monitoring for all outpatients. Web a new patient status form will document absolute. Healthcare providers must be certified in the clozapine rems to prescribe for outpatient use. A new patient status form (psf) will be used to document absolute. Web the clozapine rems resulted from the fda amendments act of 2007 which gave the fda power to require rems programs. The form is used to document the monitoring frequency, anc values, adverse events related to clozapine. Web new patient status form to document monitoring for all outpatients. Web we encourage pharmacists and prescribers to continue working with the clozapine rems to complete certification and prescribers should continue to enroll. Patient monitoring must still continue per the. Web a new patient status form will document absolute neutrophil count (anc) monitoring for all outpatients. This form must be submitted monthly. The patient status form (psf), and the. Web the new patient status is used to document monitoring of the anc. Web after a rigorous screening process, 75 publications were included in the review, which focused on three main aspects: Web patient’s clozapine rems monitoring frequency. Web beginning on november 15, the clozapine rems requires a new patient status form to document monitoring for all outpatients. To receive treatment, a patient must be enrolled in the clozapine rems program by a certified doctor. This form must be submitted monthly. This form must be submitted monthly. Beginning november 15, 2021, prescribers must complete and submit the patient. Web to add a patient status form, click the add psf button on the appropriate patient’s row. It's simple to start saving today at the pharmacy.Clozapine 100 Mg Tablet, Vega Biotec Pvt Ltd, 10x10, ID 23516355497
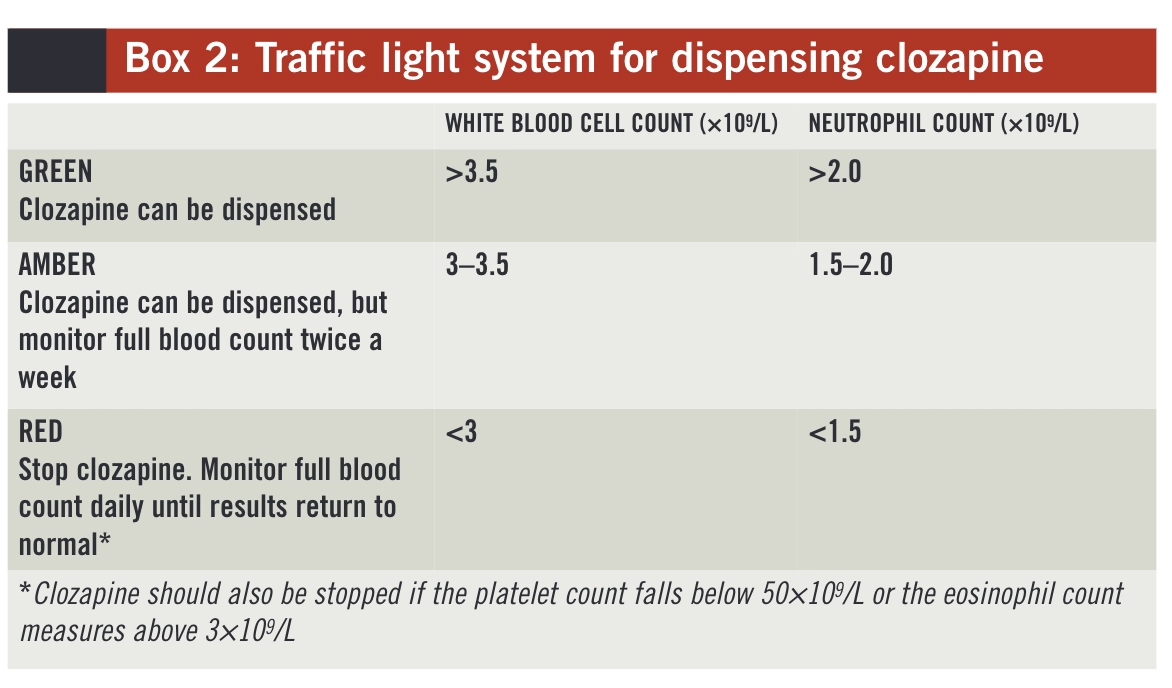
Clozapine blood monitoring for side effects of agranulocytosis
Spravato Rems Patient Enrollment Form Enrollment Form
Clozapine Simple and Practical Mental Health
Clozapine Tablets(PSYCLOZE) CONSERN PHARMA
How clozapine patients can be monitored safely and effectively The
Clozapine Tablets IP 50 MG., Prescription, Rs 53.50 /stripe Dellwich
Medication card Clozapine ACTIVE LEARNING TEMPLATES
PPT Antipsychotics and Physical Healththe Clozapine Resources
Clozapine Prescriptiongiant
Related Post: