Oxygen Electron Configuration Long Form
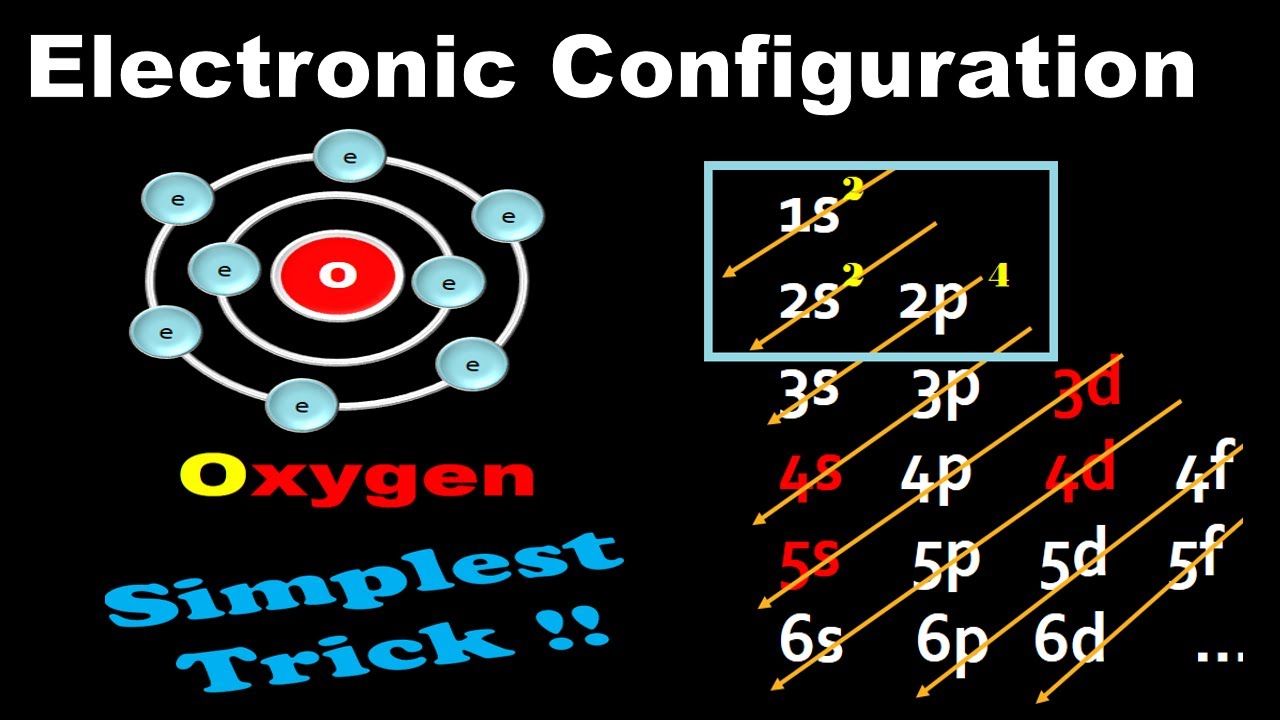
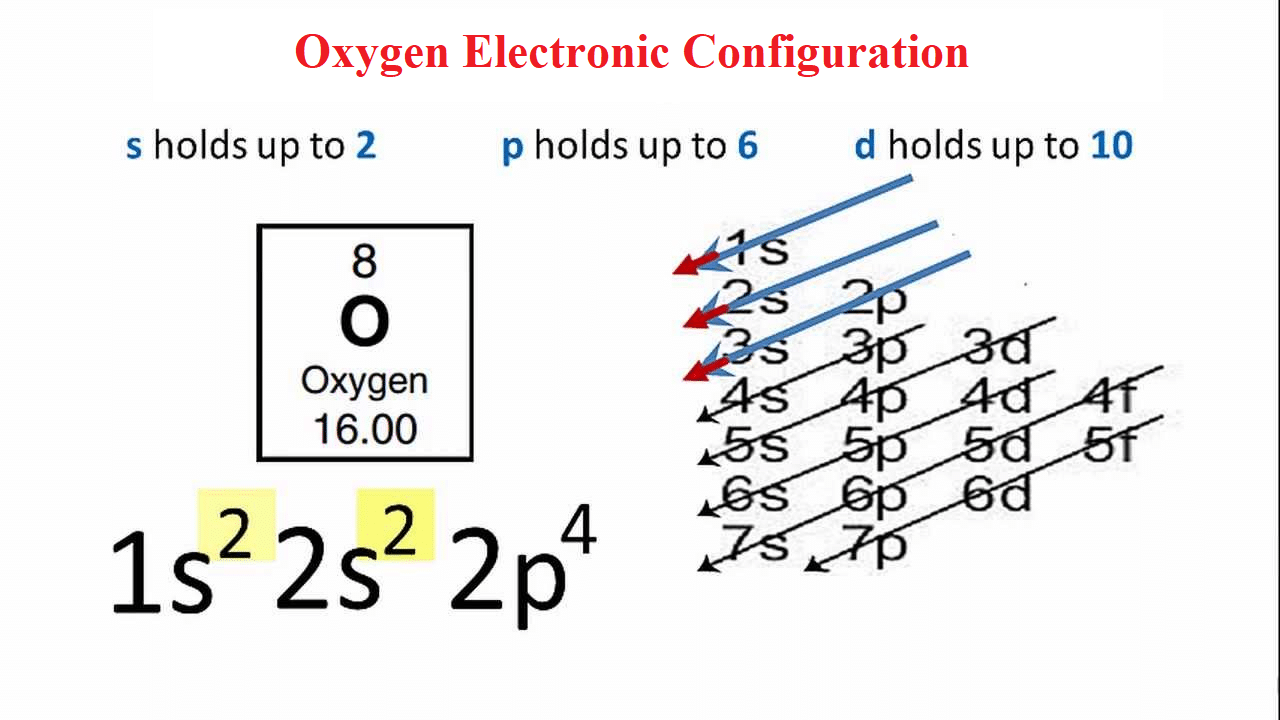
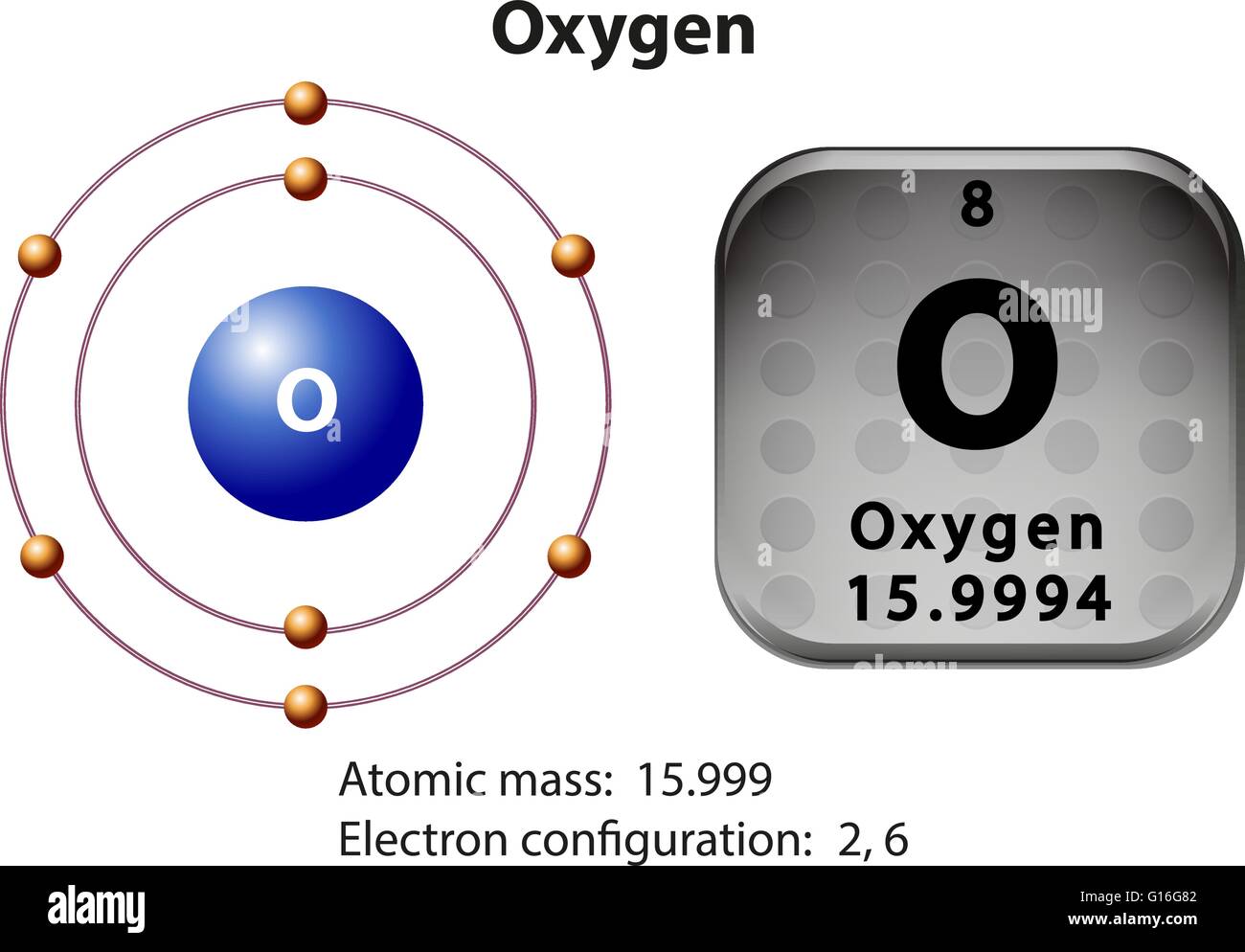
Oxygen Electron Configuration Long Form - Web what is the long form of oxygen’s electron configuration? Web next, consider oxygen (z = 8) atom, the element after nitrogen in the same period; Web oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single. An atom’s valence is its ability to combine. Web these three electrons have unpaired spins. 1 s 2 2 s 2 2 p 4. To know more about the atomic structure of oxygen, you need to learn about the electronic. Let's find the electron configuration of oxygen! Web for example, the electron configuration of oxygen looks like: Since 1s can only hold two electrons the next 2 electrons for o go in the 2s orbital. To know more about the atomic structure of oxygen, you need to learn about the electronic. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single. Oxygen. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Web therefore, if two electrons occupy the same orbital, they must have different spins. Web electron configuration [he]2s 2 2p 4: Web what is the long form of oxygen’s electron configuration? Web the electron configuration and the orbital diagram are: Let's find the electron configuration of oxygen! Web next, consider oxygen (z = 8) atom, the element after nitrogen in the same period; This notation shows the distribution of all 8 electrons of. Web therefore, if two electrons occupy the same orbital, they must have different spins. Web for example, the electron configuration of oxygen looks like: Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single. Web oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. A single oxygen atom has 8 protons and 8 electrons, but how. An atom’s valence is its ability to combine. The chemical symbol for oxygen is o. We describe an electron configuration with a symbol that contains three. The remaining four electrons will go in the 2p orbital. Web an oxygen atom has: The long form of oxygen’s electron configuration is 1s² 2s² 2p⁴. Web oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. Web an oxygen atom has: 1s 2 2s 2 2p 4. To know more about the atomic structure of oxygen, you need to learn about the. 1s 2 2s 2 2p 4. The name oxygen was created. Let's find the electron configuration of oxygen! Web as a result, the o electron configuration will be 1s2, 2s2, 2p4 1 s 2, 2 s 2, 2 p 4. Web these three electrons have unpaired spins. Since it is the outermost (valence) electrons which are. Oxygen has one more electron. Web the electron configuration and the orbital diagram are: 1 s 2 2 s 2 2 p 4. To know more about the atomic structure of oxygen, you need to learn about the electronic. Web oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. The configuration notation provides an easy way for scientists to write and. An atom’s valence is its ability to combine. Web as a result, the o electron configuration will be 1s2, 2s2, 2p4 1 s 2, 2. Web electron configuration [he]2s 2 2p 4: Web note that the last term in the oxygen electron configuration will be 1s2 2s2 2p4. Oxygen has one more electron. Web therefore, if two electrons occupy the same orbital, they must have different spins. This notation shows the distribution of all 8 electrons of. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Let's find the electron configuration of oxygen! The configuration notation provides an easy way for scientists to write and. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single. Since it is the outermost (valence) electrons which are. The long form of oxygen’s electron configuration is 1s² 2s² 2p⁴. Web note that the last term in the oxygen electron configuration will be 1s2 2s2 2p4. An atom’s valence is its ability to combine. The chemical symbol for oxygen is o. Oxygen has one more electron. Web oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. The name oxygen was created. Web oxygen electron configuration long form. This notation shows the distribution of all 8 electrons of. A single oxygen atom has 8 protons and 8 electrons, but how do we know. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. 1s 2 2s 2 2p 4. 1 s 2 2 s 2 2 p 4. This is the reason all orbitals can hold a maximum of two electrons.Electronic Configuration For Oxygen spdf Trick Chemistry Atomic
Oxygen Electron Configuration How to Write the Electron Configuration
Electron Configuration Of Oxygen Diagram worksheet
oxygen18 Element information Neutrons, Protons, Atom, Oxygen
Electronic Configuration for Oxygen What's Insight
Symbol and electron diagram for Oxygen illustration Stock Vector Image
Oxygen Art Print by Carlos Clarivan Atomic structure, Atom, Electron
Molecular Orbital Diagrams for O2 101 Diagrams
Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
What is the Electron Configuration of Oxygen Archives Dynamic
Related Post: