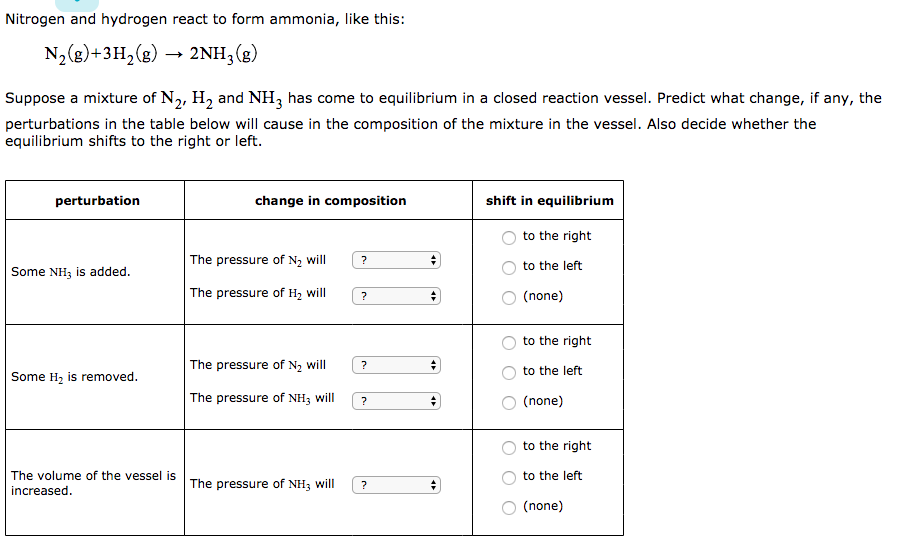
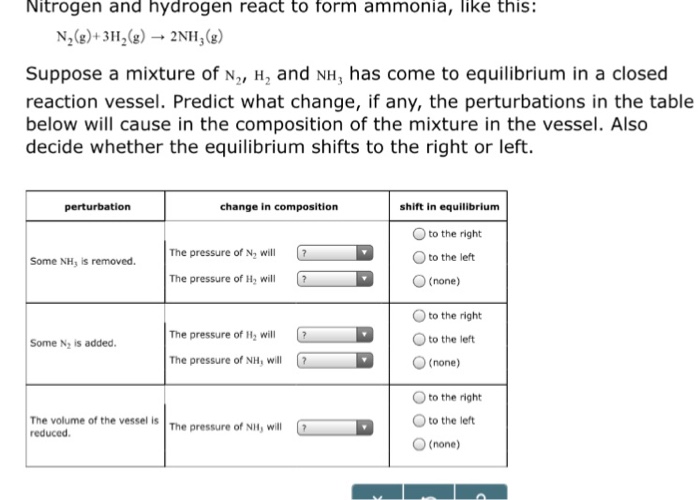
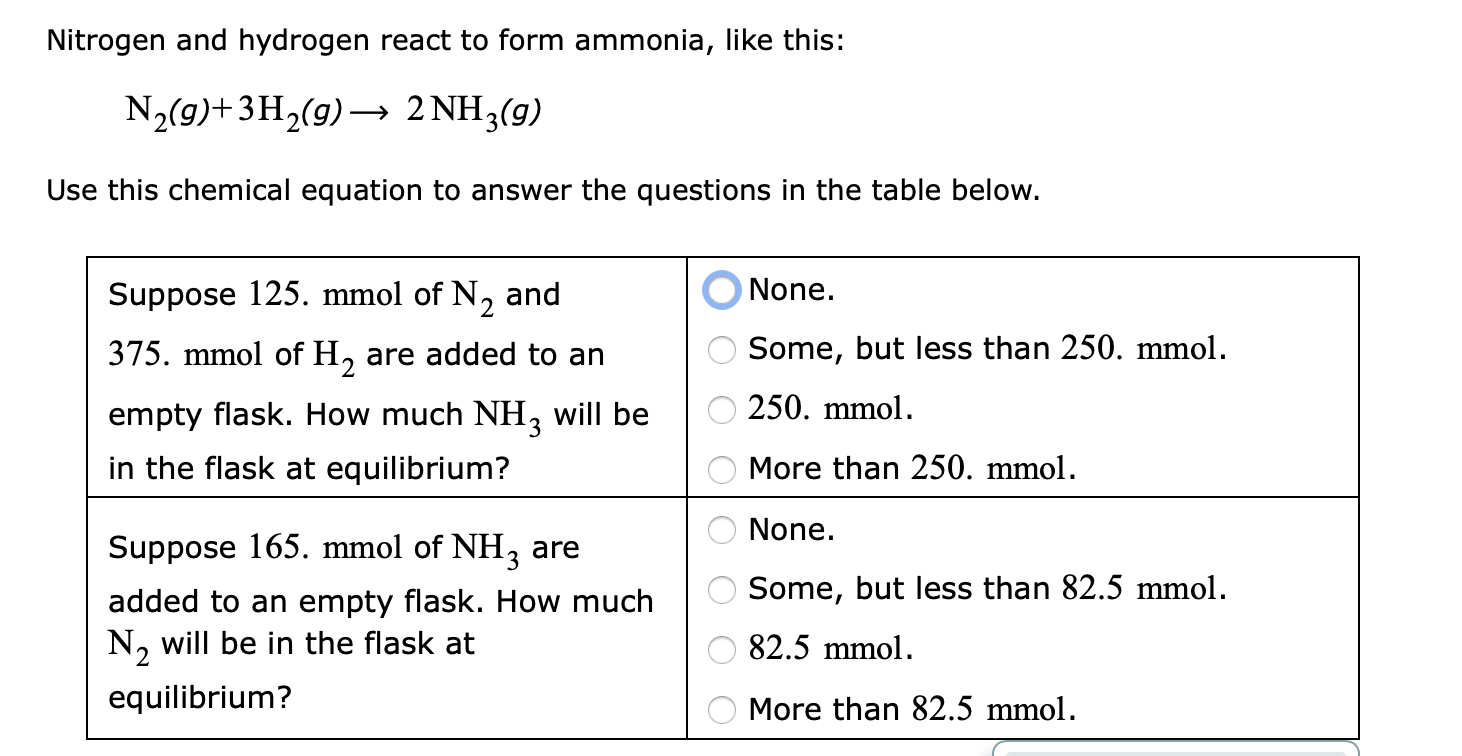
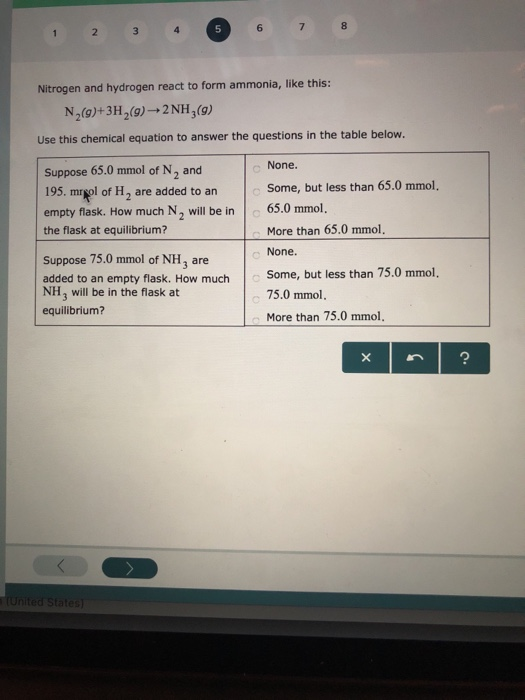
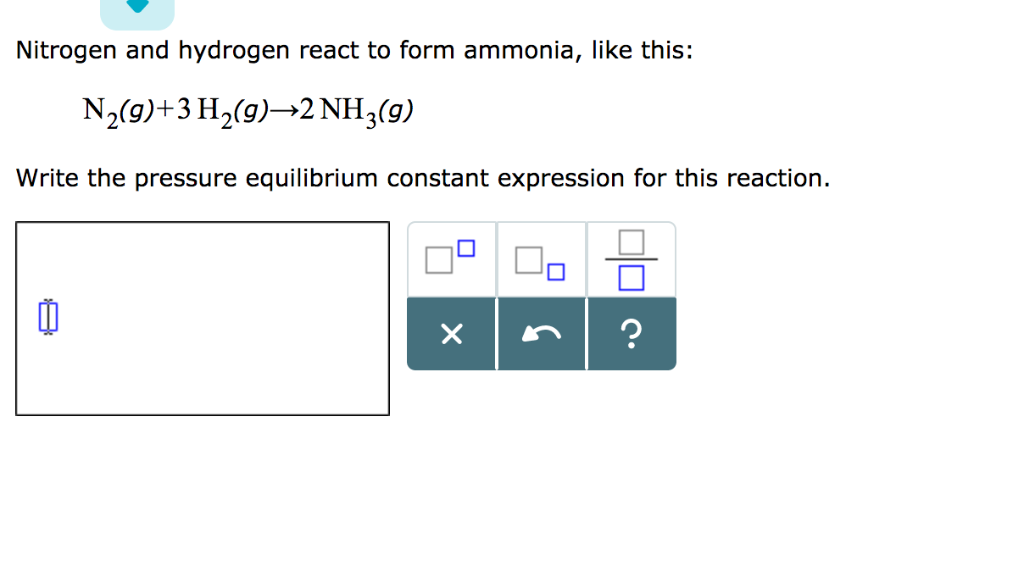
Nitrogen And Hydrogen React To Form Ammonia
Nitrogen And Hydrogen React To Form Ammonia - If 4.0 moles of h2 with 2.0 moles of n2 are reacted, how many. When hydrogen gas combines with nitrogen to form ammonia the following chemical reaction will take place. Nitrogen and hydrogen react to form ammonia. Web nitrogen and hydrogen combine to form ammonia in the haber process. 2n2 + 3h2 → 2nh3 nitrogen and hydrogen gases react to form ammonia. Web the given chemical reaction is; N2 (g) + 3h2 (g) → 2nh3 (g) calculate the number of moles of hydrogen. Web nitrogen and hydrogen react to form ammonia. Web the reaction of nitrogen with hydrogen to form ammonia is thermodynamically favorable. N2 (g) +3h2 (g) →2nh3 (g) imagine 119.mmol of nh3 are added to an empty flask, and then answer the following. Hence, if nitrogen and hydrogen. Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2 (g)+3h2 (g) + 2nh3 (g) suppose a mixture of n2, h, and nh, has come to equilibrium in a closed reaction. N2 (g) + 3h2 (g) rightarrow 2nh3 (g) use. N2 (g) +3h2 (g) →2nh3 (g) imagine 119.mmol of nh3. The reaction mixture contains some ammonia, plus a lot of unreacted nitrogen and hydrogen. Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: Web nitrogen and hydrogen react to form ammonia, like this: Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of ammonia.. Web nitrogen and hydrogen react to form ammonia. Nitrogen and hydrogen react to form ammonia. A) 3.0 mol n2 and 5.0 mol h2. Web nitrogen and hydrogen combine to form ammonia in the haber process. Web the given chemical reaction is; Web nitrogen and hydrogen react to form ammonia, like this: Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: The reaction mixture contains some ammonia, plus a lot of unreacted nitrogen and hydrogen. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. Nitrogen gas is. A) 3.0 mol n2 and 5.0 mol h2. Web nitrogen and hydrogen react to form ammonia. Determine the limiting reactant for each combination of reactants. Web the reaction of nitrogen with hydrogen to form ammonia is thermodynamically favorable. If 4.0 moles of h2 with 2.0 moles of n2 are reacted, how many. The standard enthalpy change (δh°) for a reaction can be. N2 (g) +3h2 (g) →2nh3 (g) imagine 119.mmol of nh3 are added to an empty flask, and then answer the following. N2 + 3h2 → 2nh3. Web nitrogen and hydrogen react to form ammonia, like this: Nitrogen and hydrogen react to form ammonia. N2 (g) + 3h2 (g) → 2nh3 (g) calculate the number of moles of hydrogen. Web nitrogen and hydrogen combine to form ammonia in the haber process. 2n2 + 3h2 → 2nh3 nitrogen and hydrogen gases react to form ammonia. N2 + 3h2 ⇌ 2nh3. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 +. The mixture is cooled and. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. Web the given chemical reaction is; N2 (g)+3h2 (g) + 2nh3 (g) suppose a mixture of n2, h, and nh, has come to equilibrium in a closed reaction. N2 (g) +3h2 (g) →2nh3 (g) imagine 119.mmol. Web the reaction of nitrogen with hydrogen to form ammonia is thermodynamically favorable. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of ammonia. Web nitrogen and hydrogen react to form ammonia, like this: Our equilibrium reaction will be n2(g) +. The reaction mixture contains some ammonia, plus a. \mathrm {n_2} (g)+3\;\mathrm {h_2} (g)\rightarrow 2\;\mathrm {nh_3} (g). Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 2 nh3 if 4.0 moles of h2 with 2.0 mol of n2 are reacted, how many moles of. N2(g) + 3h2(g) → 2nh3(g) identify the limiting reactant (hydrogen or nitrogen) in each of the following combinations. What is the reaction to form ammonia? Web nitrogen and hydrogen react to form ammonia, like this: N2 (g) + 3h2 (g) rightarrow 2nh3 (g) use. The mixture is cooled and. Web nitrogen and hydrogen react to form ammonia. N2 + 3h2 → 2nh3. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. Web nitrogen and hydrogen react to form ammonia, like this: The reaction mixture contains some ammonia, plus a lot of unreacted nitrogen and hydrogen. Web the membrane increases the efficiency of the plasma process because it limits the regeneration of ammonia from decomposition products. When hydrogen gas combines with nitrogen to form ammonia the following chemical reaction will take place. N2 (g)+3h2 (g) + 2nh3 (g) suppose a mixture of n2, h, and nh, has come to equilibrium in a closed reaction. Our equilibrium reaction will be n2(g) +. Nitrogen gas is triple bonded. A) 3.0 mol n2 and 5.0 mol h2. \mathrm {n_2} (g)+3\;\mathrm {h_2} (g)\rightarrow 2\;\mathrm {nh_3} (g). Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 2 nh3 if 4.0 moles of h2 with 2.0 mol of n2 are reacted, how many moles of. Hence, if nitrogen and hydrogen. Web nitrogen and hydrogen react to form ammonia: Web the reaction of nitrogen with hydrogen to form ammonia is thermodynamically favorable.Solved Nitrogen and hydrogen react to form ammonia, like
[Solved] Hydrogen gas and nitrogen gas can react to form ammonia
Solved Nitrogen and hydrogen react to form ammonia, like
Solved Nitrogen and hydrogen react to produce ammonia
Solved Nitrogen and hydrogen react to form ammonia, like
Solved 6 7 3 Nitrogen and hydrogen react to form ammonia,
Solved Nitrogen and hydrogen react to form ammonia, like
Solved Nitrogen and hydrogen react to form ammonia, like
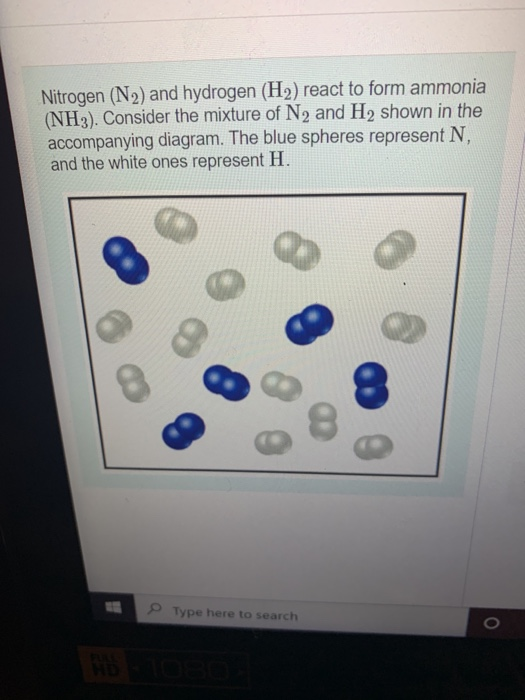
Solved Nitrogen (N2) and hydrogen (H2) react to form ammonia
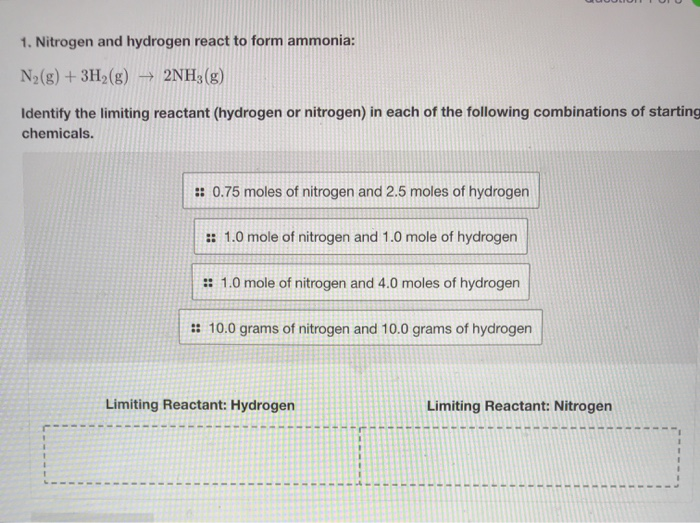
Solved 1. Nitrogen and hydrogen react to form ammonia N2(g)
Related Post: