Neon Electron Configuration Long Form
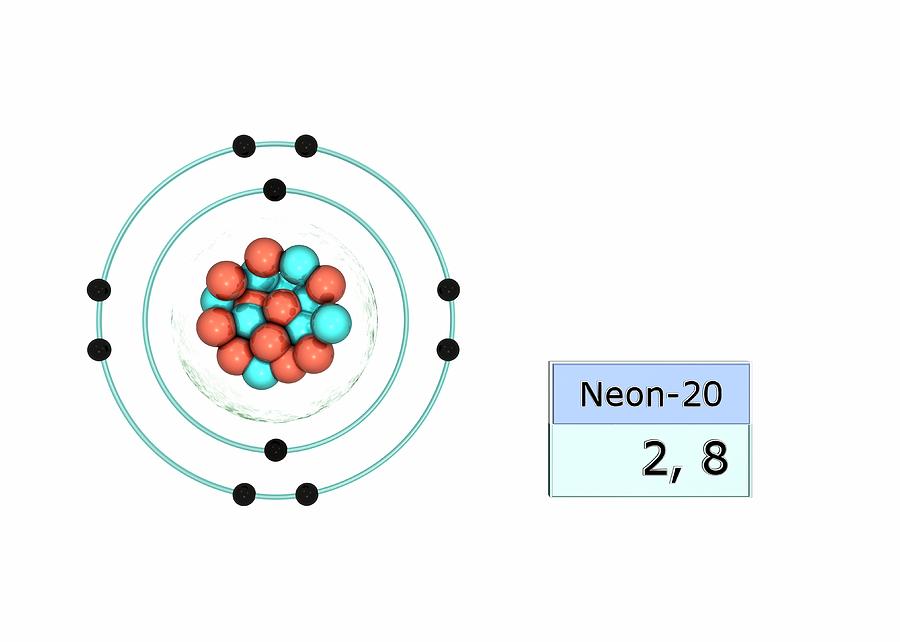
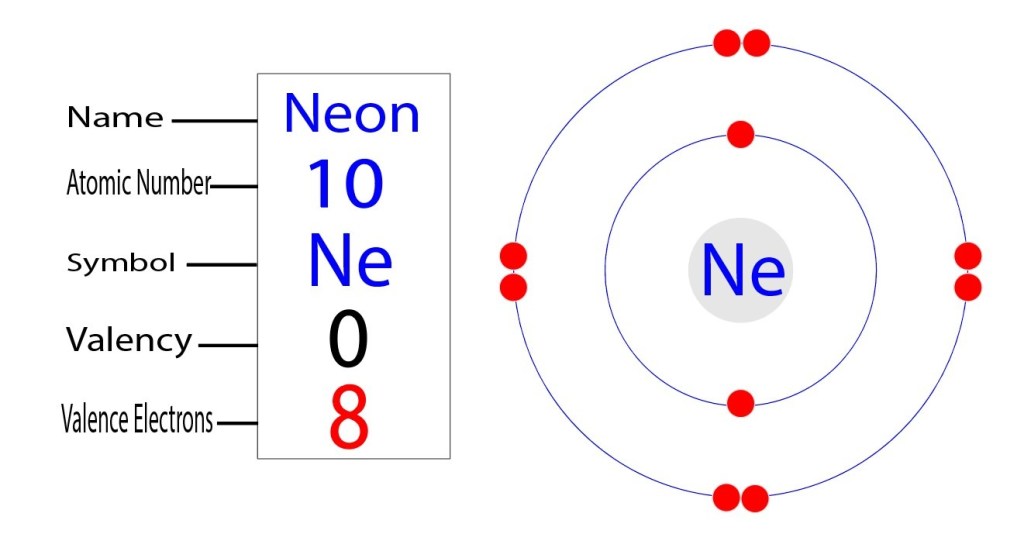
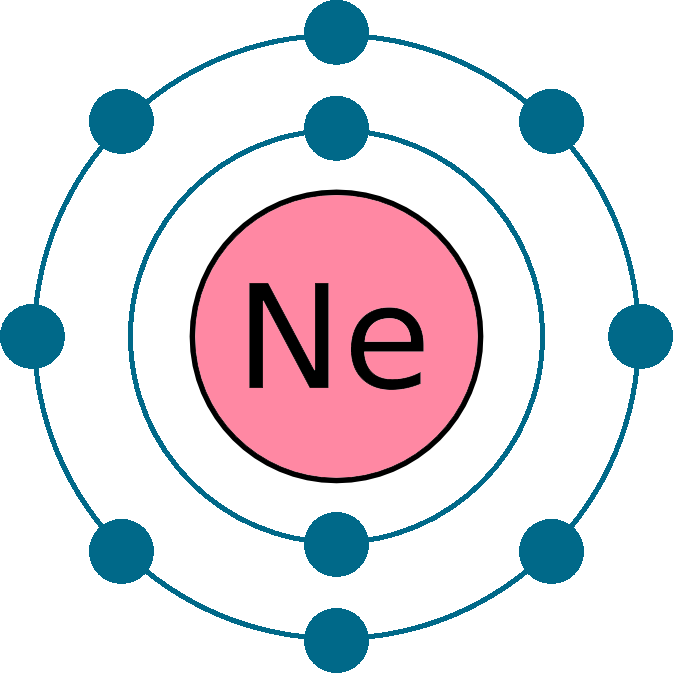
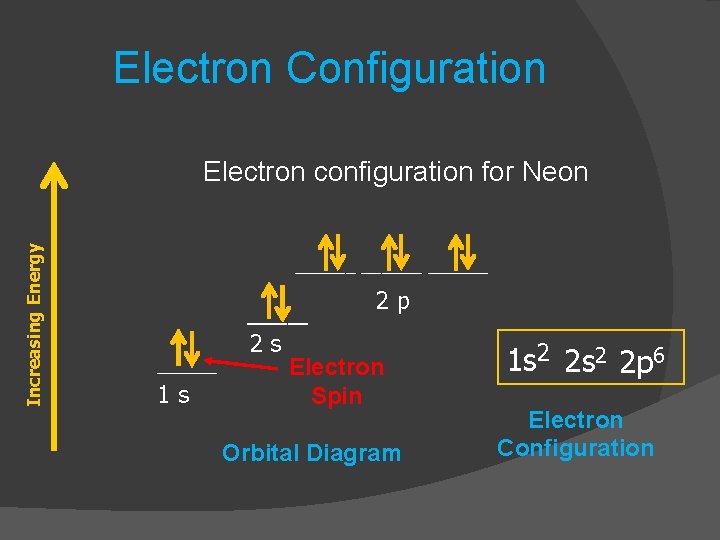
Neon Electron Configuration Long Form - 1s 2 2s 2 2p 1. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Electron configuration can be done in two ways. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. The electron configuration of neon is 2s 2 2p 6, if the electron arrangement is through orbitals. Web electron configurations of ions. The electron configurations and orbital diagrams of these four elements are: 1 s 2 2 s 2. This configuration indicates that neon has a completely filled outer electron shell, making it stable and unreactive. Web electron configuration of carbon (c) [he] 2s 2 2p 2: Web neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure.the chemical symbol for neon is ne. Hence, we can. The electron configuration of neon is 1s^2 2s^2 2p^6, which means it has a total of 10 electrons. The commonly used long form of the periodic table is designed to emphasize electron configurations. 1s 2 2s 2 2p 5: The electron configurations and orbital diagrams of these four elements are: For each atom the subshells are given first in concise. 1s 2 2s 2 2p 1. Web the electron configuration of boron is: 1 s 2 2 s 1 2 p 1. For example, to find the configuration for the lithium ion (li⁺), start with neutral lithium (1s²2s¹). Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. 1s 2 2s 2 2p 3: Web in writing the electron configuration for neon the first two electrons will go in the 1s orbital. The electron configuration of neon is 1s^2 2s^2 2p^6, which means it has a total of 10 electrons. The chemical symbol for neon is ne. For each atom the subshells are given first in concise form,. The chemical symbol for neon is ne. So oxygen's electron configuration would be o 1s 2 2s 2 2p 4. The electron configuration of neon is 2s 2 2p 6, if the electron arrangement is through orbitals. This page shows the electron configurations of the neutral gaseous atoms in their ground states. Web the electron configuration for ca 2+ is. Web electron configurations are a simple way of writing down the locations of all of the electrons in an atom. 1 s 2 2 s 1 2 p 1. The chemical symbol for neon is ne. Web electron configuration 2s 2 2p 6: Electron configuration can be done in two ways. The electron configuration of neon is 1s^2 2s^2 2p^6, which means it has a total of 10 electrons. 1 s 2 2 s 1 2 p 1. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Web electron configuration 2s 2 2p 6: So oxygen's electron configuration would be o 1s 2 2s 2 2p 4. The configuration notation provides an easy way for scientists to write and communicate how electrons are. 24.56 k (−248.59 °c, −415.46 °f) boiling point: 24.556 k, 43.37 kpa : 1s 2 2s 2 2p 4: 1 s 2 2 p 2. Web in writing the electron configuration for neon the first two electrons will go in the 1s orbital. Web the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. 1s 2 2s 2 2p 4: The process is necessary as it breaks out the whole element and makes it super easier for the. Neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. 1s 2 2s 2 2p 2: 24.556 k, 43.37 kpa : Web the electron configuration for ca 2+ is the same as that for argon, which has 18 electrons. Web the electron configuration of boron is: Web electron configuration of carbon (c) [he] 2s 2 2p 2: Neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. So oxygen's electron configuration would be o 1s 2 2s 2 2p 4. 1 s 2 2 p 2. 1 s 2 2 s 2. 1 s 2 1 p 2. When liquid (at b.p.) 1.207 g/cm 3 : For example, to find the configuration for the lithium ion (li⁺), start with neutral lithium (1s²2s¹). The electron configurations and orbital diagrams of these four elements are: 1 s 2 2 p 2. Web electron configurations of ions. Web neon is a chemical element with the symbol ne and atomic number 10. Web the electron configuration of boron is: Notice that for neon, as for helium, all the orbitals through the 2 p level are completely filled. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. 24.556 k, 43.37 kpa : The process is necessary as it breaks out the whole element and makes it super easier for the purpose of study and research. The electron configuration of neon is 2s 2 2p 6, if the electron arrangement is through orbitals. Electron configuration and oxidation states of neon. The electron configuration of neon is 1s^2 2s^2 2p^6, which means it has a total of 10 electrons.Neon Electron Configuration YouTube
Neon Electron Configuration Photograph by Photo
Diagram representation element neon Royalty Free Vector
Orbital Diagram For Neon (Ne) Neon Electron Configuration
Electron Configuration Of Neon Long Form worksheet today
Neon Atom Science Notes and Projects
PPT Orbital Filling Electron Configurations PowerPoint Presentation
Neon Element (Ne 10) of Periodic Table Periodic Table FlashCard
Neon electronic configurationhow to Write Neon electronic
Electron Configuration Of Neon Meaning worksheet
Related Post: