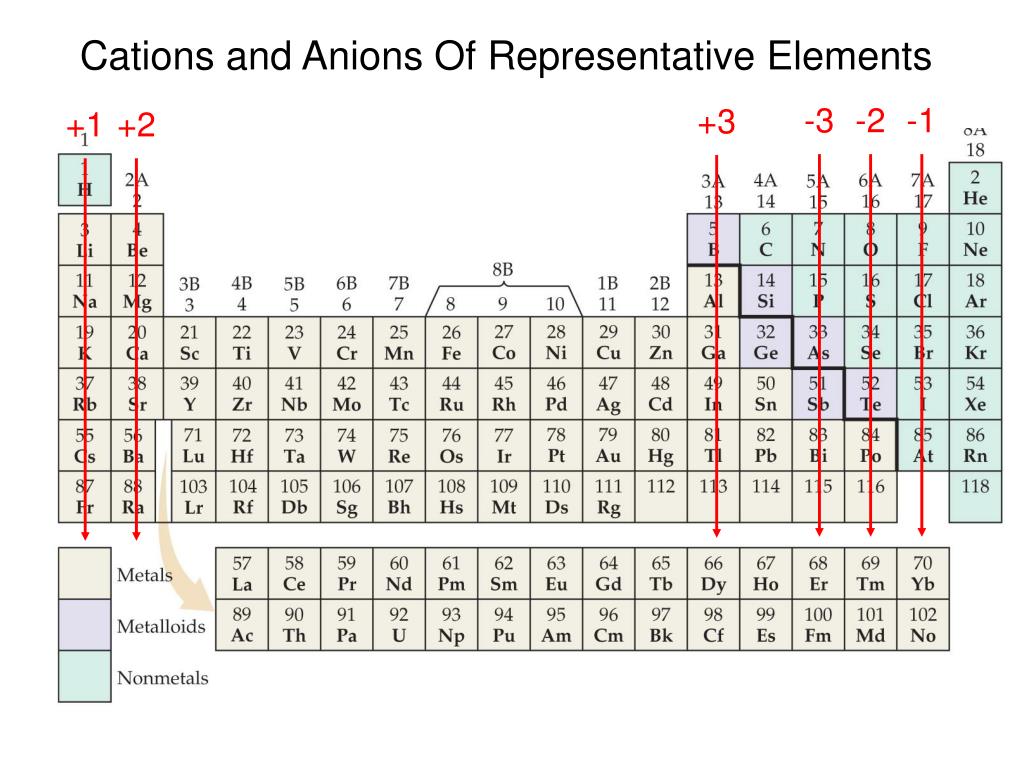
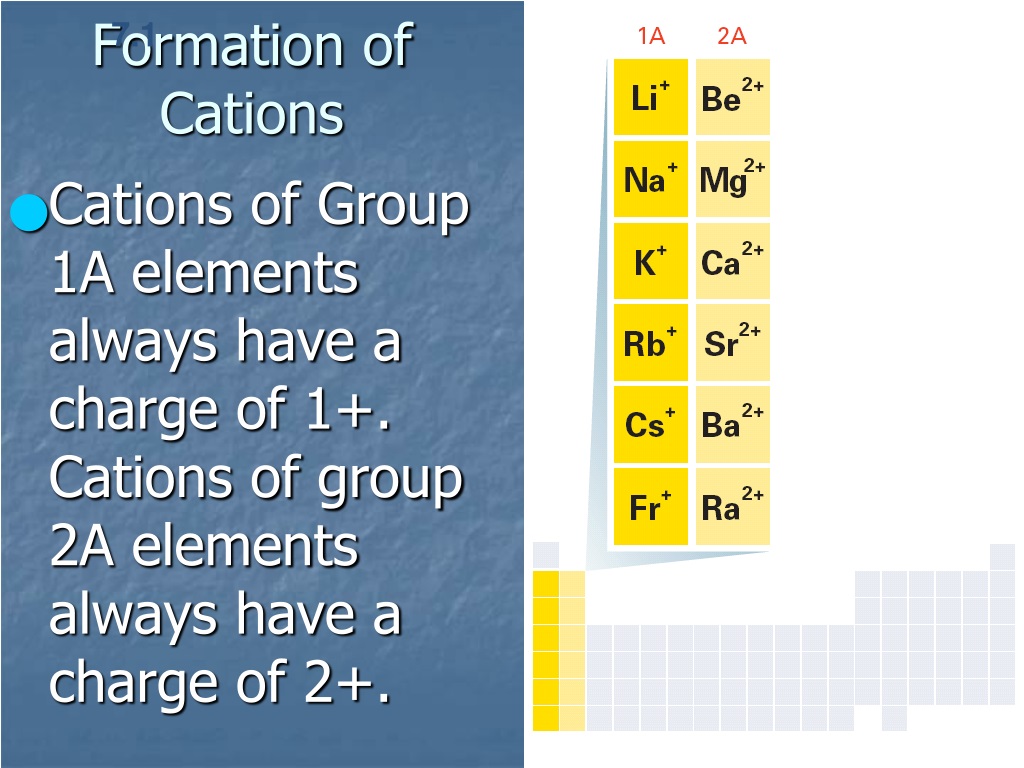
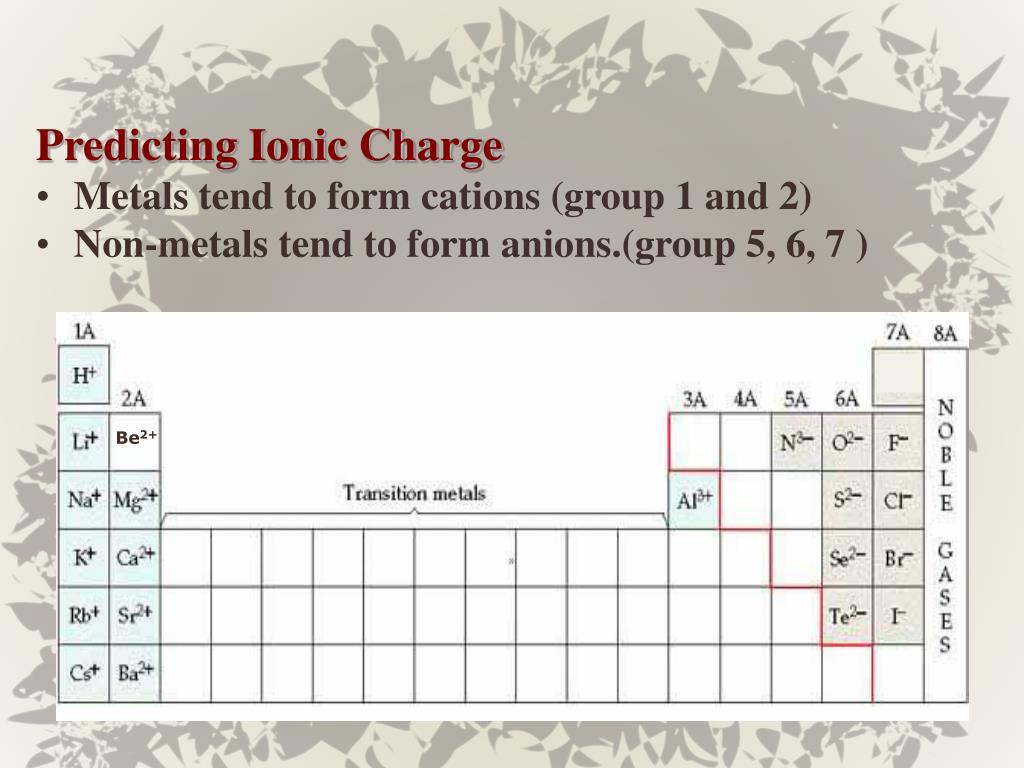
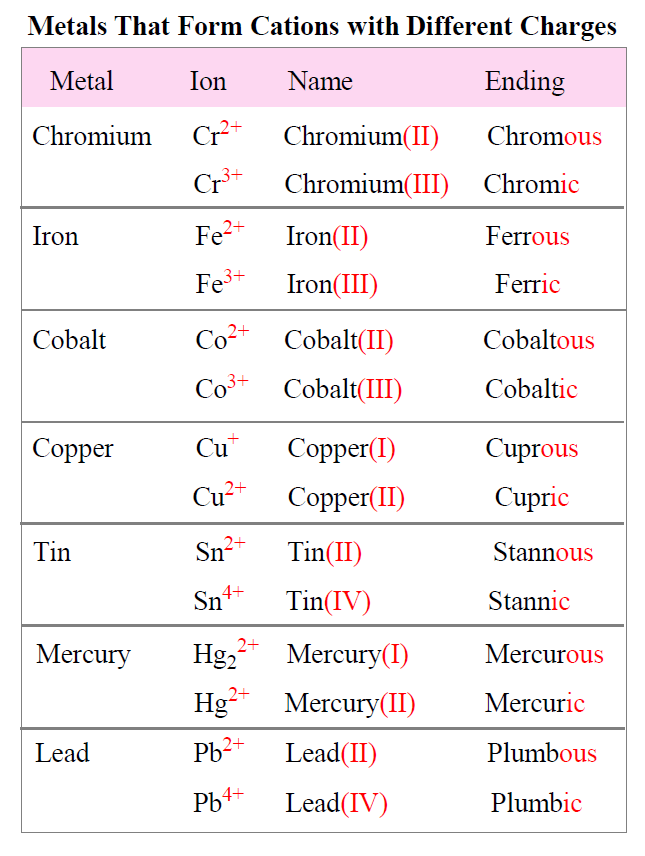
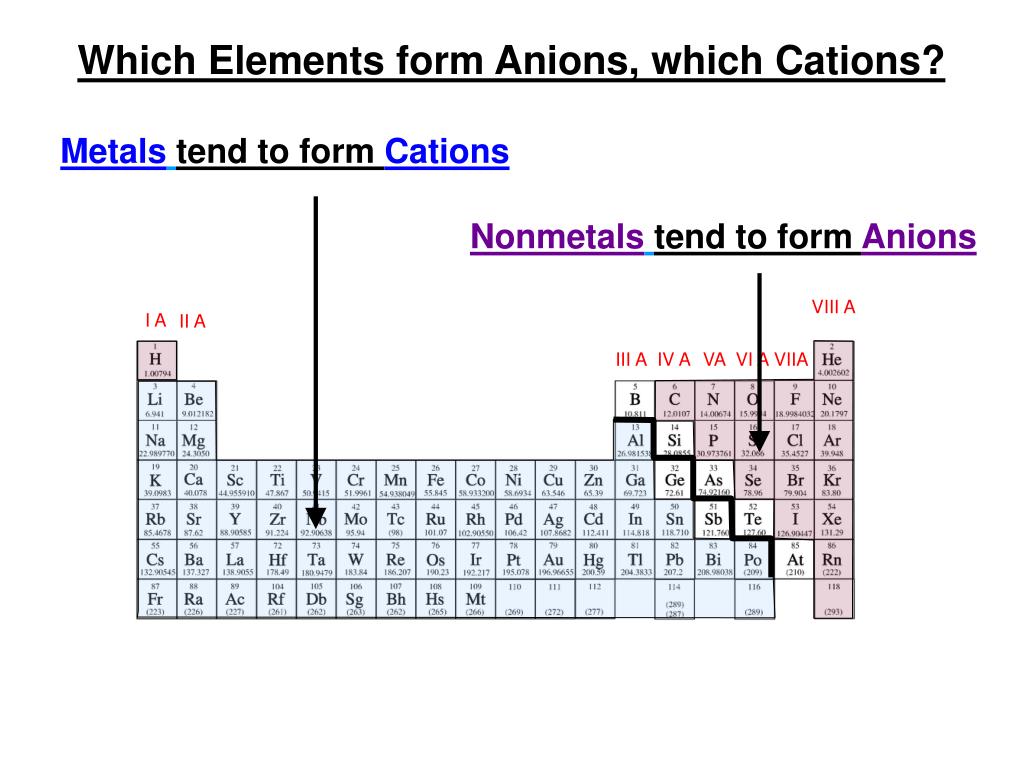
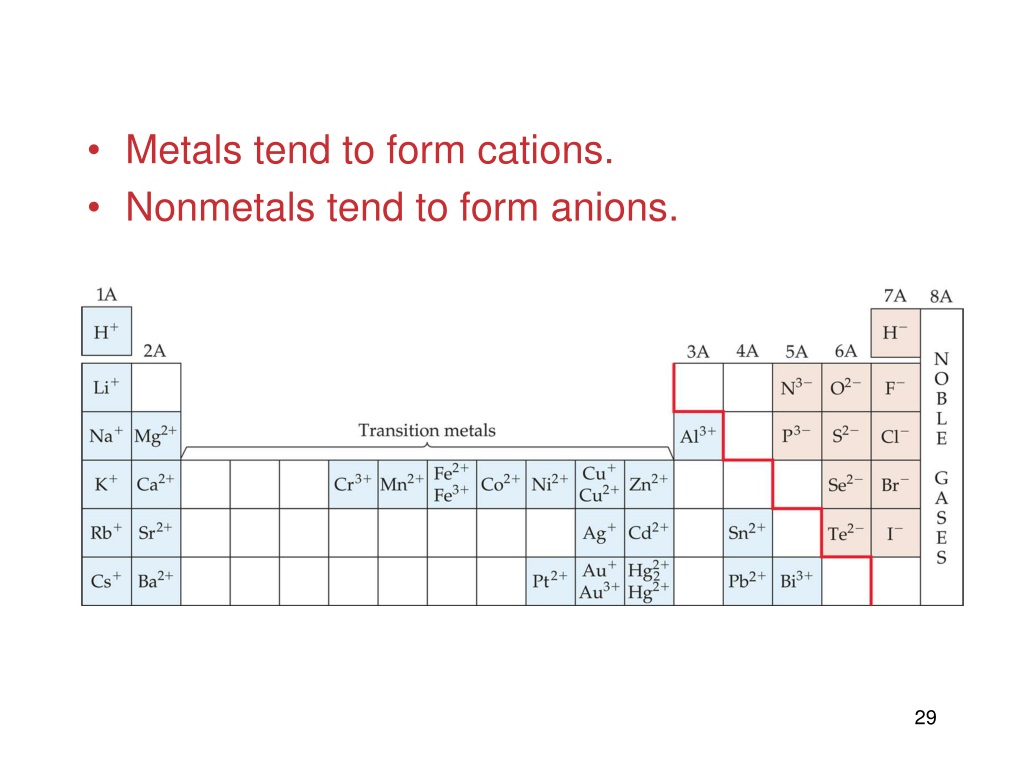
Metals Tend To Form Cations
Metals Tend To Form Cations - Web the answer is usually a metal. At least at first glance, it's not an element that suggests metallic properties. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. An atoms is usually larger than its corresponding cation. Web a) nonmetals tend to gain electrons. Web 7 rows the chemistry of the alkali metals reflects their tendency to form +1 cations. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web anions and cations 1) metals tend to _____ electrons and nonmetals tend to _____ electrons. Web we would like to show you a description here but the site won’t allow us. Web metals tend to form cations. Web the answer is usually a metal. Then, there's hydrogen, a colorless and odorless gas. Metals tend to form cations, while nonmetals tend to form anions. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which. \[\ce{na2o(s) + h2o(l) \rightarrow 2naoh(aq)}\label{1.4} \] \[\ce{cao(s) + h2o(l) \rightarrow. Anions elements that are classified as. Most metal oxides are basic oxides and dissolve in water to form metal hydroxides: Metals tend to form cations, while nonmetals tend to form anions. Web we would like to show you a description here but the site won’t allow us. A magnesium atom must lose two electrons to have the same number electrons. Web the answer is usually a metal. Web metals form positive ions (cations). A cation loses electrons causing the ion to have a positive charge. Most metal oxides are basic oxides and dissolve in water to form metal hydroxides: Web metals tend to form cations. Web the answer is usually a metal. Web metals form positive ions (cations). Web anions and cations 1) metals tend to _____ electrons and nonmetals tend to _____ electrons. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web can you explain why metals tend to. \[\ce{na2o(s) + h2o(l) \rightarrow 2naoh(aq)}\label{1.4} \] \[\ce{cao(s) + h2o(l) \rightarrow. Energy is released when electrons are removed from metal ions. Web elements that are classified as metals tend to form cations. Metals lose or donate their valence electrons and form cations while non. Web anions and cations 1) metals tend to _____ electrons and nonmetals tend to _____ electrons. Then, there's hydrogen, a colorless and odorless gas. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Most metal oxides are basic oxides and dissolve in water to form metal hydroxides: Anions elements that are classified as. Web the answer is. Web anions and cations 1) metals tend to _____ electrons and nonmetals tend to _____ electrons. Web metals form positive ions (cations). Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. This is actually one of the chemical properties. Most metal. Anions elements that are classified as. Most metal oxides are basic oxides and dissolve in water to form metal hydroxides: Web can you explain why metals tend to form cations, while nonmetals tend to form anions. Web metals form positive ions (cations). Web a) nonmetals tend to gain electrons. Web 7 rows the chemistry of the alkali metals reflects their tendency to form +1 cations. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which. Anions elements that are classified as. Web. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web a) nonmetals tend to gain electrons. Metals lose or donate their valence electrons and form cations while non. This is actually one of the chemical properties. Web metals form positive ions (cations). Web a) nonmetals tend to gain electrons. At least at first glance, it's not an element that suggests metallic properties. This is actually one of the chemical properties. Most metal oxides are basic oxides and dissolve in water to form metal hydroxides: Web anions and cations 1) metals tend to _____ electrons and nonmetals tend to _____ electrons. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Nonmetals tend to gain electrons. Web the answer is usually a metal. Metals tend to form cations, while nonmetals tend to form anions. Energy is released when electrons are removed from metal ions. A cation loses electrons causing the ion to have a positive charge. D) the halogens tend to form 1+ ions. Web elements that are classified as metals tend to form cations. Web metals form positive ions (cations). Web metals form positive ions (cations). \[\ce{na2o(s) + h2o(l) \rightarrow 2naoh(aq)}\label{1.4} \] \[\ce{cao(s) + h2o(l) \rightarrow. Web we would like to show you a description here but the site won’t allow us. Most metal oxides are basic oxides and dissolve in water to form metal hydroxides: Web metals tend to form cations. Metals lose or donate their valence electrons and form cations while non.Bonds From Atoms Stone Cold Chemistry Talk
PPT Mastering Chemistry PowerPoint Presentation, free download ID
PPT Chapter 7 Ionic and Metallic Bonding PowerPoint Presentation
PPT Atoms, Molecules and Ions PowerPoint Presentation, free download
Writing Chemical Formulas For Ionic Compounds Chemistry Steps
PPT Lecture 4. Chapter 2. Structure of the Atom (Contd.) PowerPoint
Cations and Anions Definitions, Examples, and Differences
Ions
PPT Chapter 7 Periodic Properties of the Elements PowerPoint
PPT 1 Name the ions formed by these elements and classify them as
Related Post: