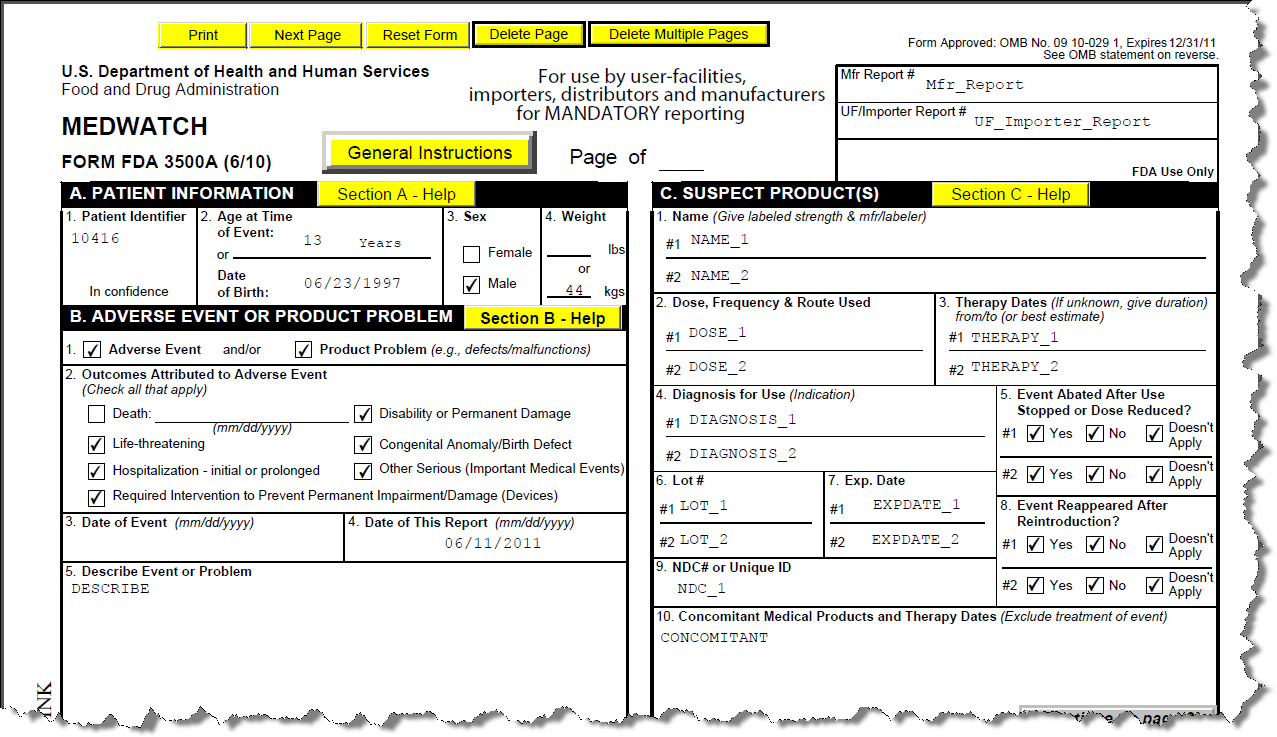
Medwatch Form 3500A
Medwatch Form 3500A - Easily fill out pdf blank, edit, and sign them. Web for form fda 3500a medwatch (for mandatory reporting) all entries should be typed or printed in a font no smaller than 8 point. Form fda 3500a (10/05) e. Medwatch form fda 3500a (mandatory reporting). Web the medwatch form, also known as form fda 3500a, is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers. 10/31/08 see omb statement on reverse. Reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: If possible, please take the 3500 form to your health professional (e.g., doctor or pharmacist) so that information based on your. Save or instantly send your ready documents. Ad download or email fda 3500a & more fillable forms, register and subscribe now! Mdr mandatory reporting, food and drug administration. Web form fda 3500 author: (hcfa or fda provided no.) (year) (sequence no.) for each report in the range of report numbers listed above, attach a completed copy of part 2 of. The fda safety information and adverse event reporting program. Web online using the medwatch online reporting form; Web medwatch as voluntary reports. Complete all sections that apply. The fda safety information and adverse event reporting program. Web online using the medwatch online reporting form; With only section d (suspect medical device). 10/31/08 see omb statement on reverse. Form fda 3500a (10/05) e. Web the medwatch form, also known as form fda 3500a, is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers. If possible, please take the 3500 form to your health professional (e.g., doctor or pharmacist) so that information based on your. Web by. Web medwatch is the fda reporting system for adverse events (aes), and form 3500a is used for adverse event reporting. Easily fill out pdf blank, edit, and sign them. Web the medwatch form, also known as form fda 3500a, is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers. 10/31/08 see omb statement on. For voluntary reporting of adverse events, product problems and product use/medication errors created date: Medwatch form fda 3500a (mandatory reporting). If possible, please take the 3500 form to your health professional (e.g., doctor or pharmacist) so that information based on your. Web medwatch as voluntary reports. Web medwatch is the fda reporting system for adverse events (aes), and form 3500a. It is for use by user facilities, distributors, importers, applicants, and manufacturers for. Web the medwatch form, also known as form fda 3500a, is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers. Ad download or email fda 3500a & more fillable forms, register and subscribe now! (hcfa or fda provided no.) (year) (sequence. Ad download or email fda 3500a & more fillable forms, register and subscribe now! If possible, please take the 3500 form to your health professional (e.g., doctor or pharmacist) so that information based on your. Web for form fda 3500a medwatch (for mandatory reporting) all entries should be typed or printed in a font no smaller than 8 point. Reporting. Save or instantly send your ready documents. Financial interests and arrangements of clinical investigators. With only section d (suspect medical device). 10/31/08 see omb statement on reverse. Form fda 3500a (10/05) e. (hcfa or fda provided no.) (year) (sequence no.) for each report in the range of report numbers listed above, attach a completed copy of part 2 of. The fda safety information and adverse event reporting program. Information for consumers, patients and caregivers. With only section d (suspect medical device). Web medwatch is the fda reporting system for adverse events (aes),. Center for devices and radiological health. (hcfa or fda provided no.) (year) (sequence no.) for each report in the range of report numbers listed above, attach a completed copy of part 2 of. General instructions for completing fda form 3500. Web the medwatch form, also known as form fda 3500a, is used for mandatory reporting of medical device adverse events. For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web the medwatch form, also known as form fda 3500a, is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers. Web form fda 3500 may also be used to submit reports about tobacco products and dietary supplements. Complete all sections that apply. Web online using the medwatch online reporting form; Mdr mandatory reporting, food and drug administration. Web on this page: Easily fill out pdf blank, edit, and sign them. Web for form fda 3500a medwatch (for mandatory reporting) all entries should be typed or printed in a font no smaller than 8 point. Save or instantly send your ready documents. It is for use by user facilities, distributors, importers, applicants, and manufacturers for. 10/31/08 see omb statement on reverse. Web medwatch as voluntary reports. (hcfa or fda provided no.) (year) (sequence no.) for each report in the range of report numbers listed above, attach a completed copy of part 2 of. If possible, please take the 3500 form to your health professional (e.g., doctor or pharmacist) so that information based on your. Financial interests and arrangements of clinical investigators. The fda safety information and adverse event reporting program. Web medwatch is the fda reporting system for adverse events (aes), and form 3500a is used for adverse event reporting. Web by standard mail: Web the medwatch form, also known as form fda 3500a, is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers.Regulatory Submissions Product Documentation
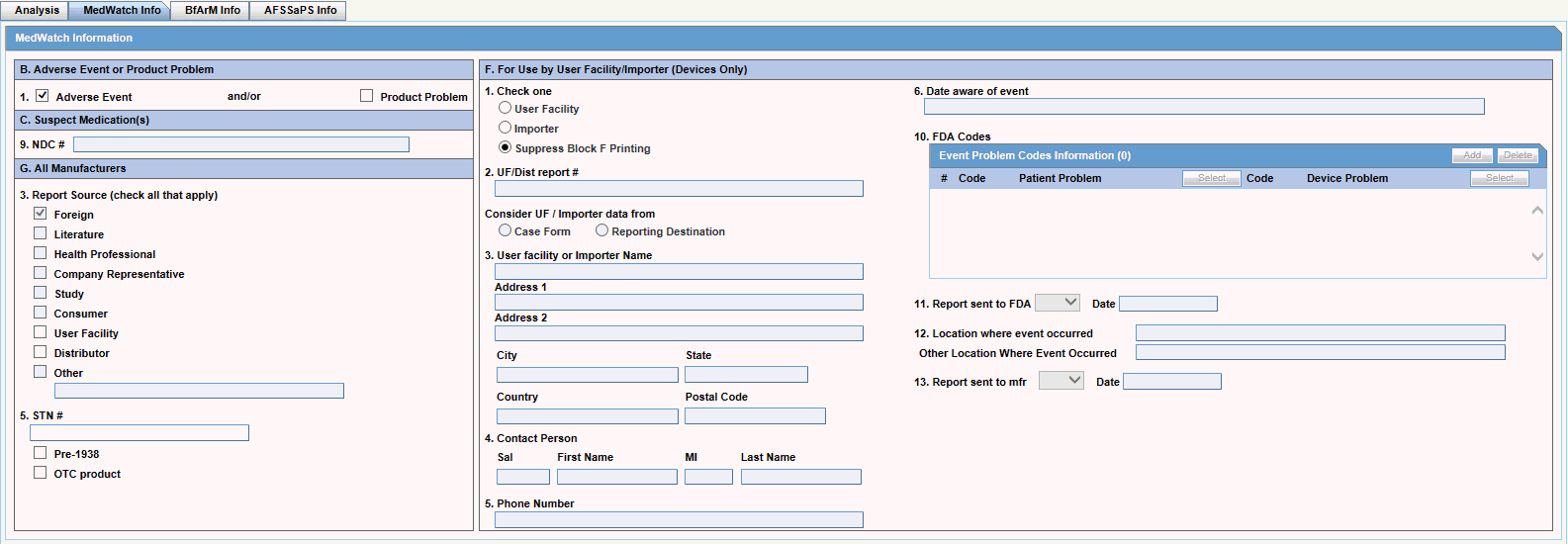
Case Form
PPT The Emerging Science of Drug Safety PowerPoint Presentation, free
Fda 3500A Form ≡ Fill Out Printable PDF Forms Online
Sources of ADR Collections and Reporting Forms Pharmacovigilance
PPT Medical Device Reporting and Tracking PowerPoint Presentation
Fillable California Form 3500a Submission Of Exemption Request
Medwatch Form Fill Out and Sign Printable PDF Template signNow
PPT Structured Data Capture (SDC) Patient Safety Event & Adverse
what is a 6k report
Related Post: