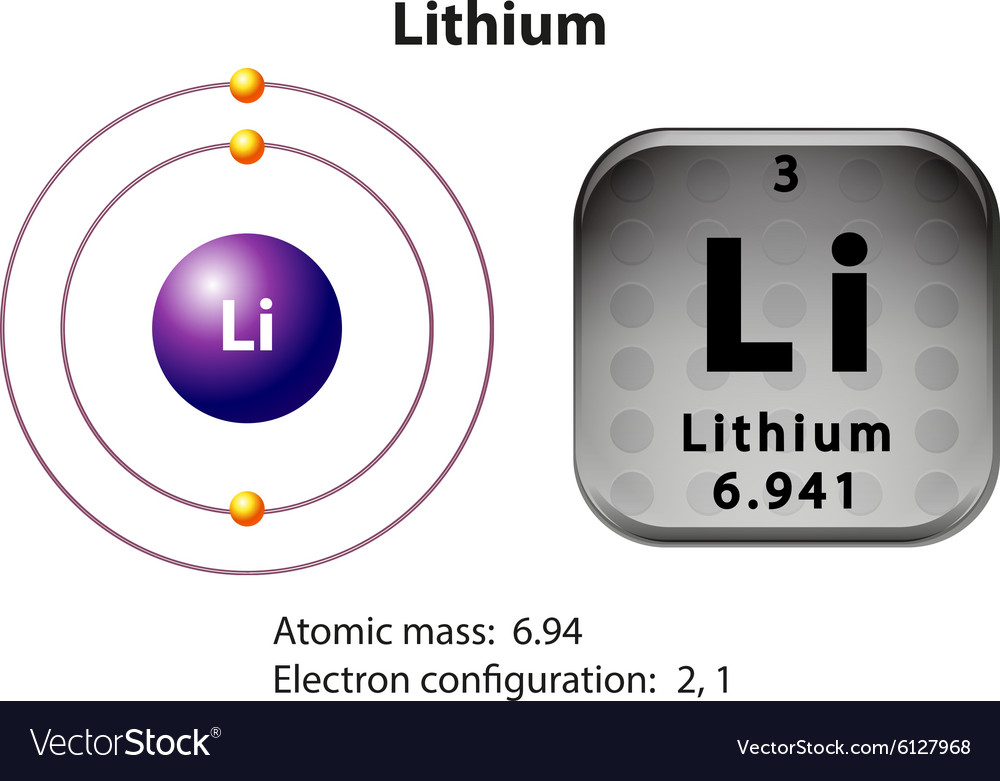
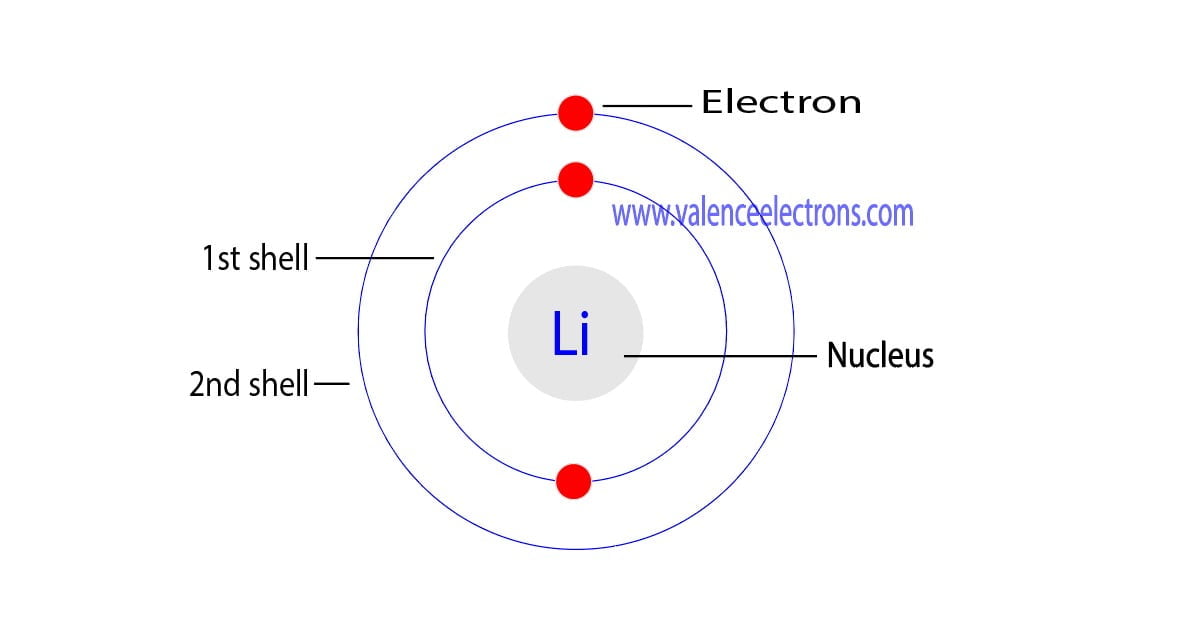
Lithium Electron Configuration Long Form
Lithium Electron Configuration Long Form - An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. Electron configuration of lithium (li) [he] 2s 1: Web electron configuration of lithium is [he] 2s1. Now, let’s take a look at the key takeaways regarding the lithium electron configuration: Web electron atomic and molecular orbitals a bohr diagram of lithium. This means that it has 2 electrons in its 1s orbital: _↑ ↓ _ _↑__ 1s 2 2s 1 Web writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Electron configuration of boron (b) [he] 2s 2 2p 1: For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. In this article, we will study how electrons are arranged in different shells and subshells in a lithium atom. We’ll also look at why lithium forms a 1+ ion and how. From its position, we know that it has 1 valence electron in the 2s orbital series (because it's in the second period): The number of the principal quantum shell,. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s and. Contact us join the conversation. Electron configuration of boron (b) [he] 2s 2 2p 1: The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l ), and. Learn more about. Web the electron configuration of lithium is:1s² 2s¹. Distribution of electrons in shell in lithium atom. Now, let’s take a look at the key takeaways regarding the lithium electron configuration: Web the electron configuration of lithium is 1s^2 2s^1, which means that it has two electrons in the 1s orbital and one electron in the 2s orbital. Web electron atomic. There are 2 electrons in an s orbital; Electron configuration of boron (b) [he] 2s 2 2p 1: Lithium is flammable, and it is potentially explosive when exposed to air and especially to water, though less so than the other alkali metals. Au5p 3/2, fe3p 1/2 binding energies of common chemical states: This means that it has 2 electrons in. We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l ), and. Web how to write the electron configuration for lithium. Web in several cases, the ground state electron configurations are different from those. We also know that its 1s orbital is full, because to get to lithium in the periodic table, we have to pass 1s. A horizontal row in the periodic table. Learn more about the occurrence and uses of lithium. There are 2 electrons in an s orbital; Au5p 3/2, fe3p 1/2 binding energies of common chemical states: The metal itself—which is soft, white, and lustrous—and several of its alloys and compounds are produced on an industrial scale. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Web. _↑ ↓ _ _↑__ 1s 2 2s 1 This configuration determines the chemical properties and behavior of lithium. In this article, we will study how electrons are arranged in different shells and subshells in a lithium atom. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Contact us join the conversation. A horizontal row in the periodic table. Web lithium is part of the group 1 alkali metals, which are highly reactive and are never found in their pure form in. The electron configuration of lithium is 2,1, which means it has only 1 electron in its valence shell out of total 3. Web lithium, chemical element of group 1 (ia) in the periodic table, the alkali metal group, lightest of the solid elements. Electron configuration of helium (he) 1s 2: An atom of the alkaline earth metal beryllium, with an. A vertical column in the periodic table. Electron configuration of lithium (li) [he] 2s 1: Electron configuration of boron (b) [he] 2s 2 2p 1: We also know that its 1s orbital is full, because to get to lithium in the periodic table, we have to pass 1s. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Web the electron configuration of lithium is:1s² 2s¹. There are 2 electrons in an s orbital; For example, the observed ground state electron configuration of chromium is [ar]4 s1 3 d5 rather than the predicted [ar]4 s2 3 d4. Learn more about the occurrence and uses of lithium. Lithium is flammable, and it is potentially explosive when exposed to air and especially to water, though less so than the other alkali metals. In this article, we will study how electrons are arranged in different shells and subshells in a lithium atom. Web in several cases, the ground state electron configurations are different from those predicted by figure 6.8.1 6.8. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s and. Since 1s can only hold two electrons the remaining electron for li goes in the 2s orbital. The shell diagram for a lithium atom (figure \(\pageindex{1}\)). Web writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. A horizontal row in the periodic table. Possible oxidation states are +1. Contact us join the conversation.【5 Steps】Electron Configuration of Lithium(Li) in Just 5 Steps
electronarrangementforlithiumatom TechnoCrazed
Lithium electronic configuration,how to Write lithium electronic
Distribution of Electrons in Different Orbits [with Examples] Teacho
Lithium Element With Reactions, Properties, Uses, & Price Periodic Table
Electron Configuration for Lithium (Li, Li+ ion)
Periodic Table Lithium Electron Configuration Periodic Table Timeline
FileElectron shell 003 Lithium.svg Wikimedia Commons Electron
Diagram representation element lithium Royalty Free Vector
Lithium(Li) electron configuration and orbital diagram (2022)
Related Post:

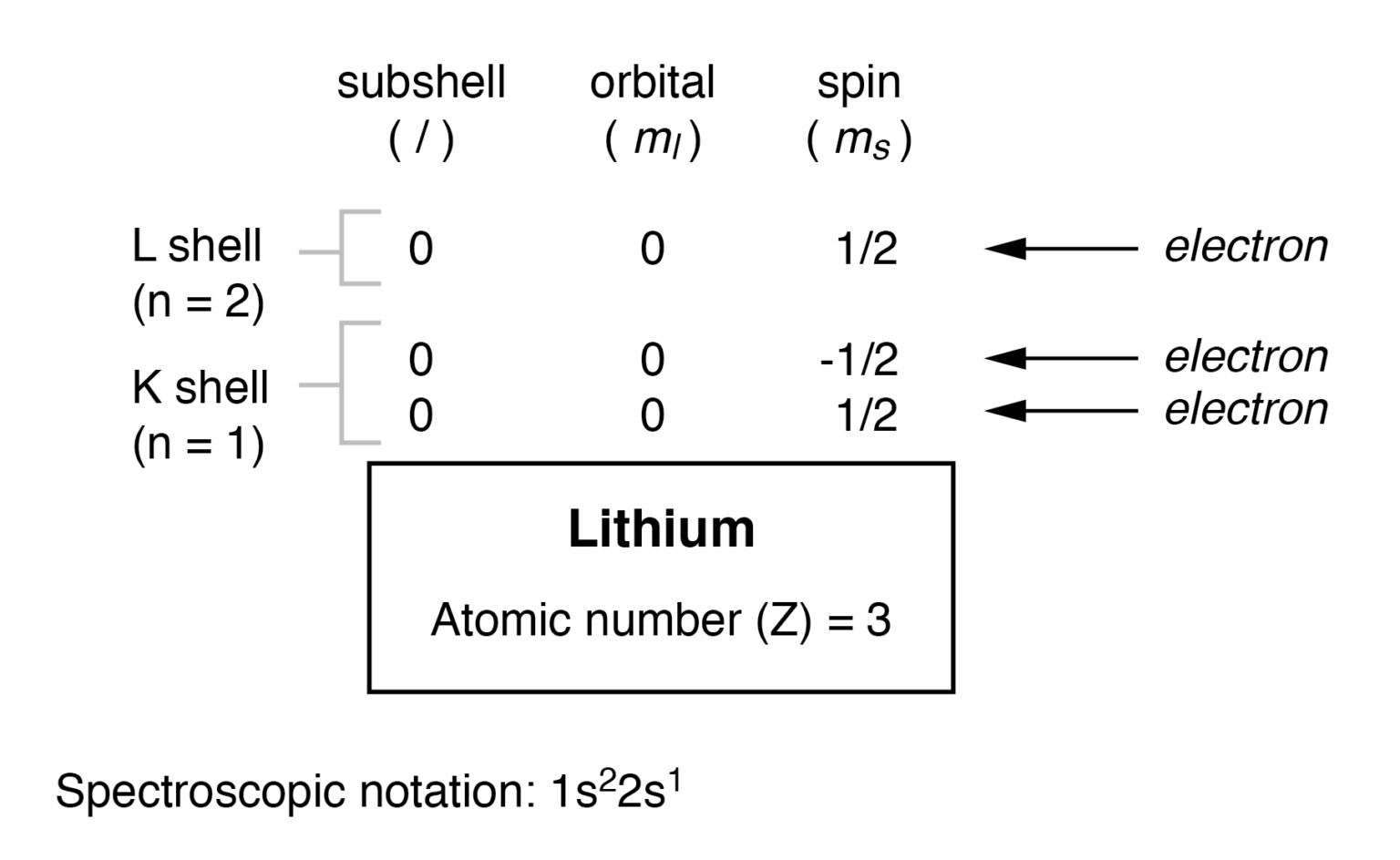

![Distribution of Electrons in Different Orbits [with Examples] Teacho](https://d1avenlh0i1xmr.cloudfront.net/021c42fa-8422-49cb-a9eb-7150c58d4610/16.-lithium-teachoo-01.png)