Keto Tautomeric Form
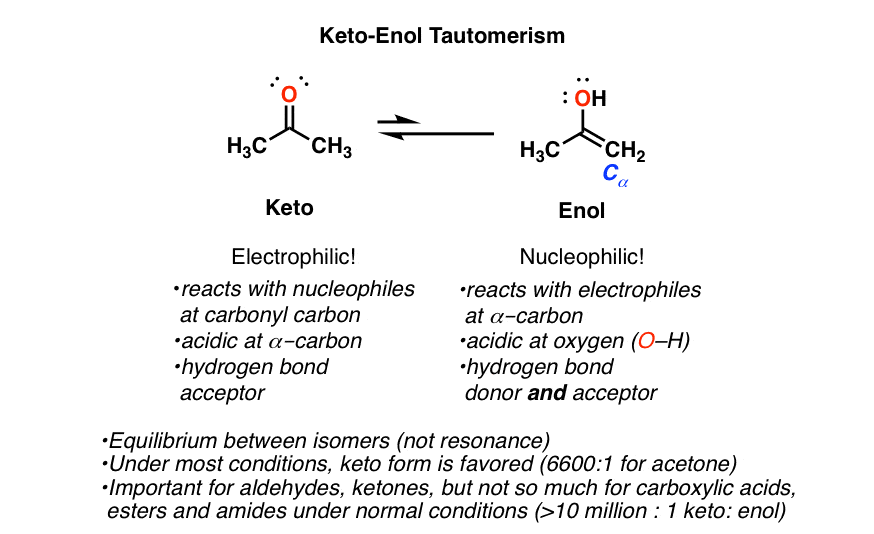
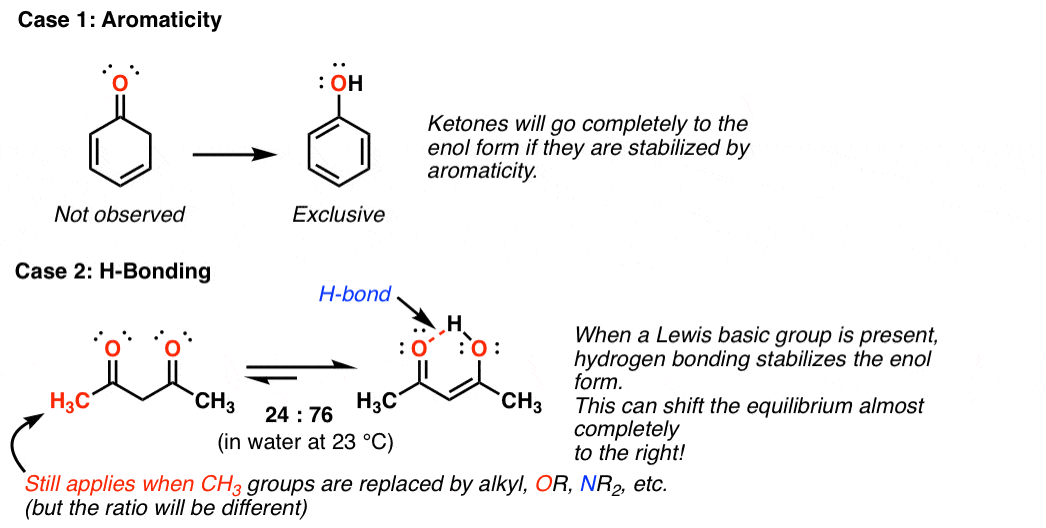
Keto Tautomeric Form - Web generally, keto tautomer is more stable than enol groups. Web in the following, we first present the quantum chemistry overview of the two tautomeric systems: (1) acetone and its enol form, and (2) edaravone’s keto, enol, and. Web and these two molecules, this ketone and this enol form, these are called tautomers. Web in both cases the “keto form” is favored by equilibrium. And the keto form is actually the much more stable form. Web uncover how aldehydes or ketones, with a dash of acid or base, can transform into an enol. Keto and enol are functional groups. In a solution, you won't see much of the. Explore the role of alpha protons and double bonds in this transformation, and understand. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). Explore the role of alpha protons and double bonds in this transformation, and understand. Web keto form definition, the form of. Explore the role of alpha protons and double bonds in this transformation, and understand. Web generally, keto tautomer is more stable than enol groups. Of course, when the hydroxyl group is attached to a cyclic structure, the “ keto form ” is always a ketone. Web and these two molecules, this ketone and this enol form, these are called tautomers.. And the keto form is actually the much more stable form. Web in the following, we first present the quantum chemistry overview of the two tautomeric systems: N the form of tautomeric compounds when. In a solution, you won't see much of the. Web in both cases the “keto form” is favored by equilibrium. N the form of tautomeric compounds when. Of course, when the hydroxyl group is attached to a cyclic structure, the “ keto form ” is always a ketone. Web in the following, we first present the quantum chemistry overview of the two tautomeric systems: In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in. Explore the role of alpha protons and double bonds in this transformation, and understand. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). And the keto form is actually the. And the keto form is actually the much more stable form. Web in both cases the “keto form” is favored by equilibrium. Web and these two molecules, this ketone and this enol form, these are called tautomers. (1) acetone and its enol form, and (2) edaravone’s keto, enol, and. Web keto form definition, the form of tautomeric compounds when they. Web uncover how aldehydes or ketones, with a dash of acid or base, can transform into an enol. In a solution, you won't see much of the. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end. Web in the following, we first present the quantum chemistry overview of the two tautomeric systems: Keto form synonyms, keto form pronunciation, keto form translation, english dictionary definition of keto form. (1) acetone and its enol form, and (2) edaravone’s keto, enol, and. Web uncover how aldehydes or ketones, with a dash of acid or base, can transform into an. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). And the keto form is actually the much more stable form. Keto and enol are functional groups. Of course, when the. Web generally, keto tautomer is more stable than enol groups. Web in the following, we first present the quantum chemistry overview of the two tautomeric systems: And the keto form is actually the much more stable form. Web and these two molecules, this ketone and this enol form, these are called tautomers. Web in both cases the “keto form” is. Web keto form definition, the form of tautomeric compounds when they are ketones rather than enols see more. N the form of tautomeric compounds when. Web generally, keto tautomer is more stable than enol groups. In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (c=c−oh). Web uncover how aldehydes or ketones, with a dash of acid or base, can transform into an enol. In a solution, you won't see much of the. Web in the following, we first present the quantum chemistry overview of the two tautomeric systems: Of course, when the hydroxyl group is attached to a cyclic structure, the “ keto form ” is always a ketone. Keto and enol are functional groups. And the keto form is actually the much more stable form. (1) acetone and its enol form, and (2) edaravone’s keto, enol, and. Web in both cases the “keto form” is favored by equilibrium. Keto form synonyms, keto form pronunciation, keto form translation, english dictionary definition of keto form. Explore the role of alpha protons and double bonds in this transformation, and understand. Web and these two molecules, this ketone and this enol form, these are called tautomers.Keto Enol Tautomerism A Summary of Key Points — Master Organic Chemistry
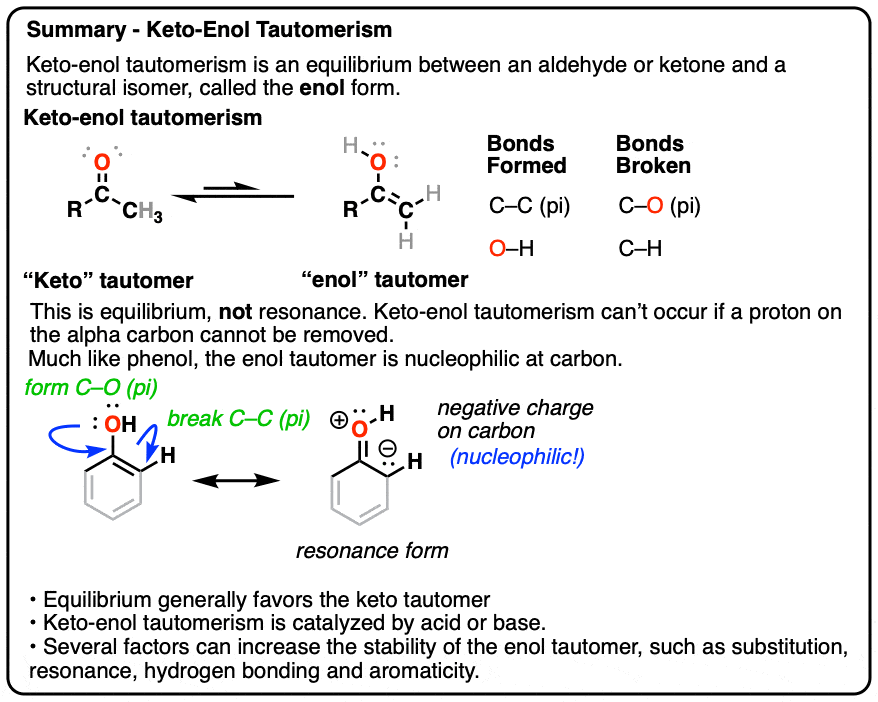
KetoEnol Tautomerism Key Points Master Organic Chemistry
Ketoenol tautomerism in HL¹ ligand Download Scientific Diagram
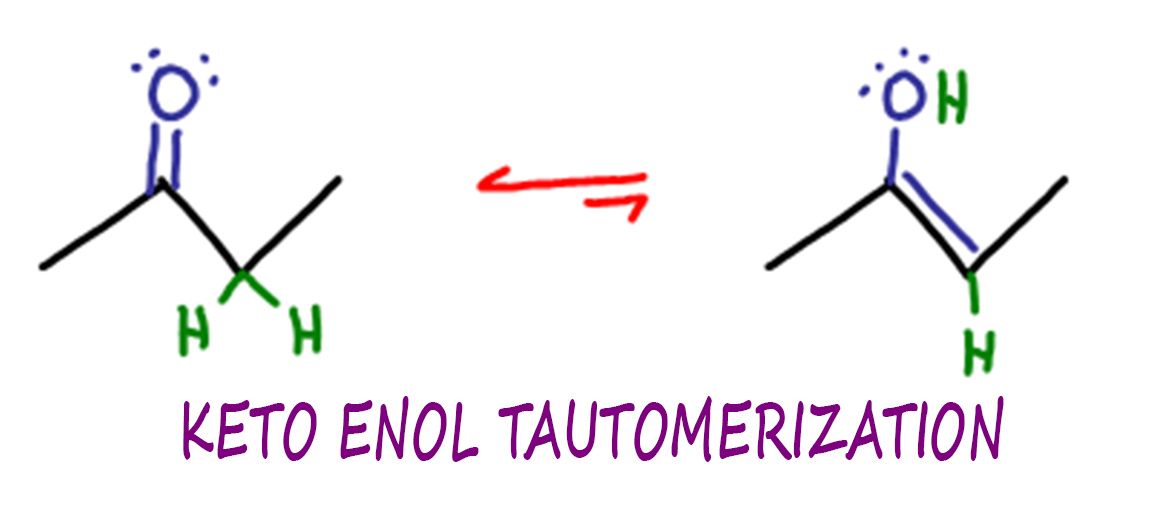
Keto Enol Tautomerization Reaction and Mechanism in Acid and Base
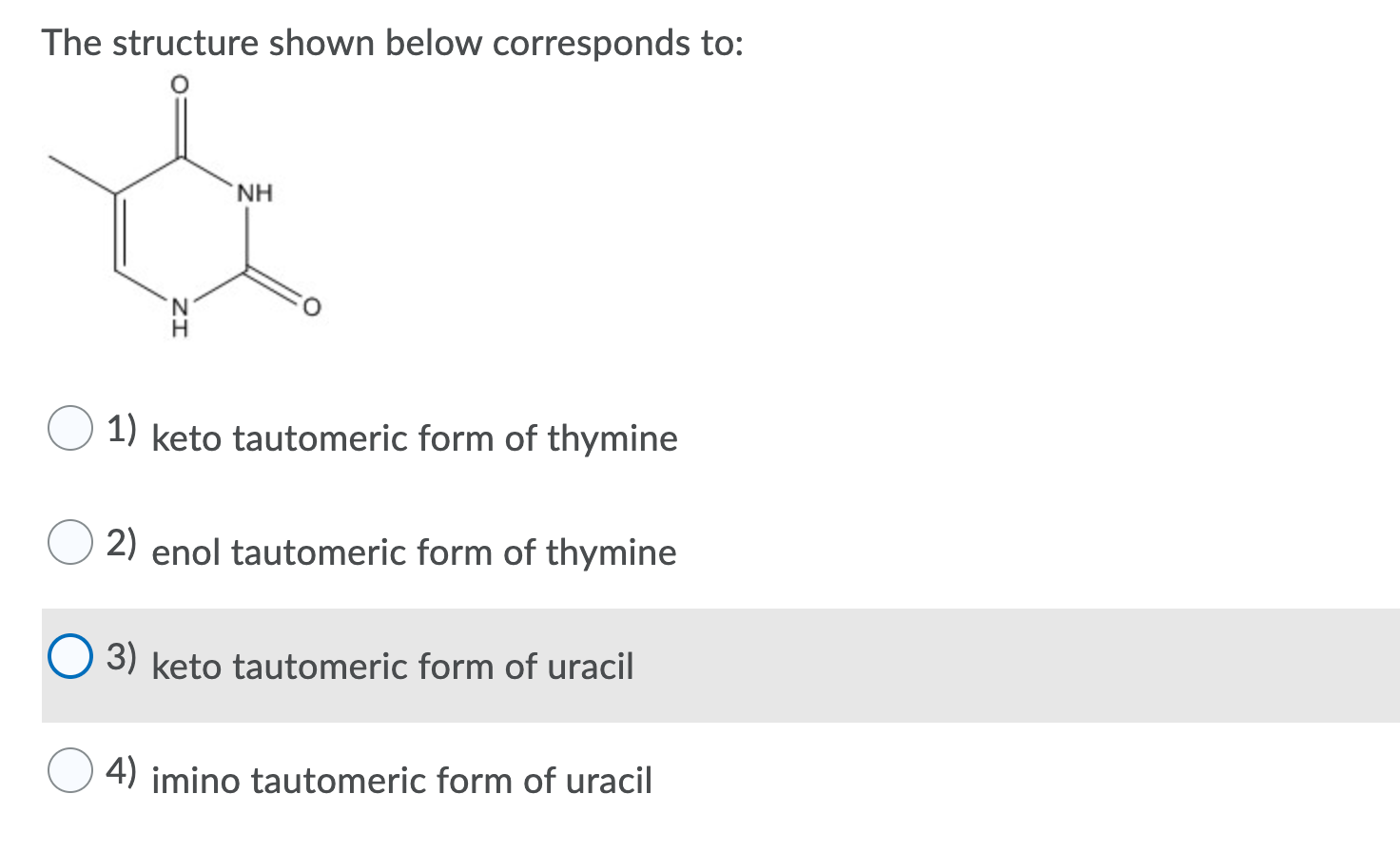
Question The Structure Shown Below Corresponds To NH 1) Keto
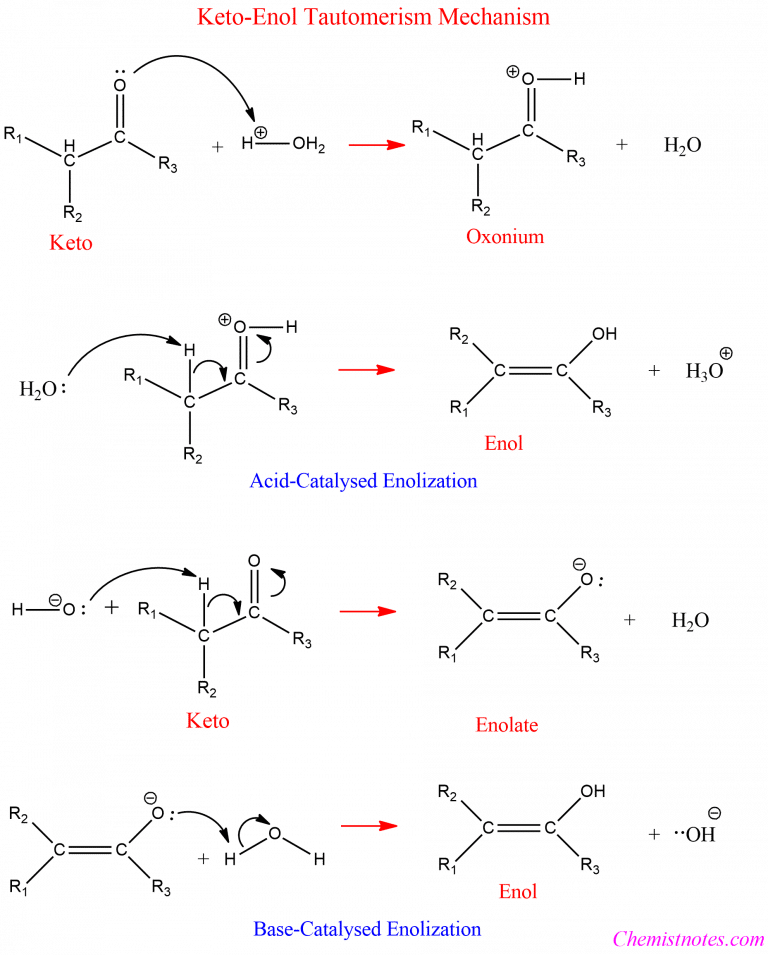
KetoEnol Tautomerism Examples, Mechanism Chemistry Notes
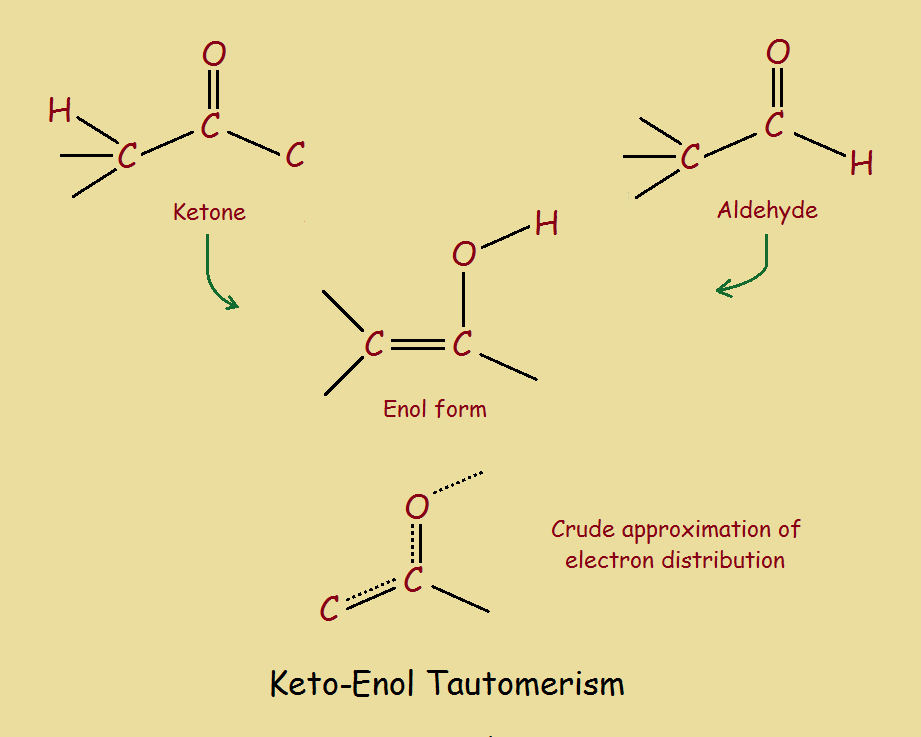
Keto Enol Tautomerism What Is It and Why Is It Important?
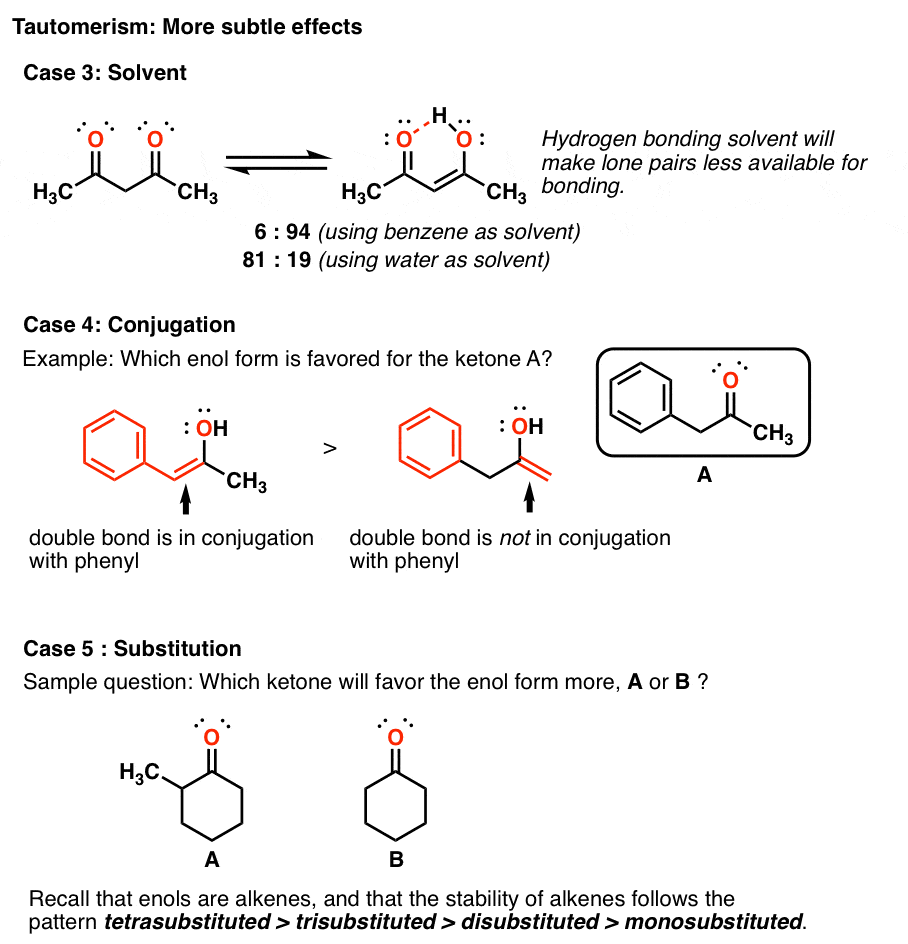
KetoEnol Tautomerism Key Points Master Organic Chemistry
Ketoenol tautomerism reaction Royalty Free Vector Image
KetoEnol Tautomerism Key Points Master Organic Chemistry
Related Post: