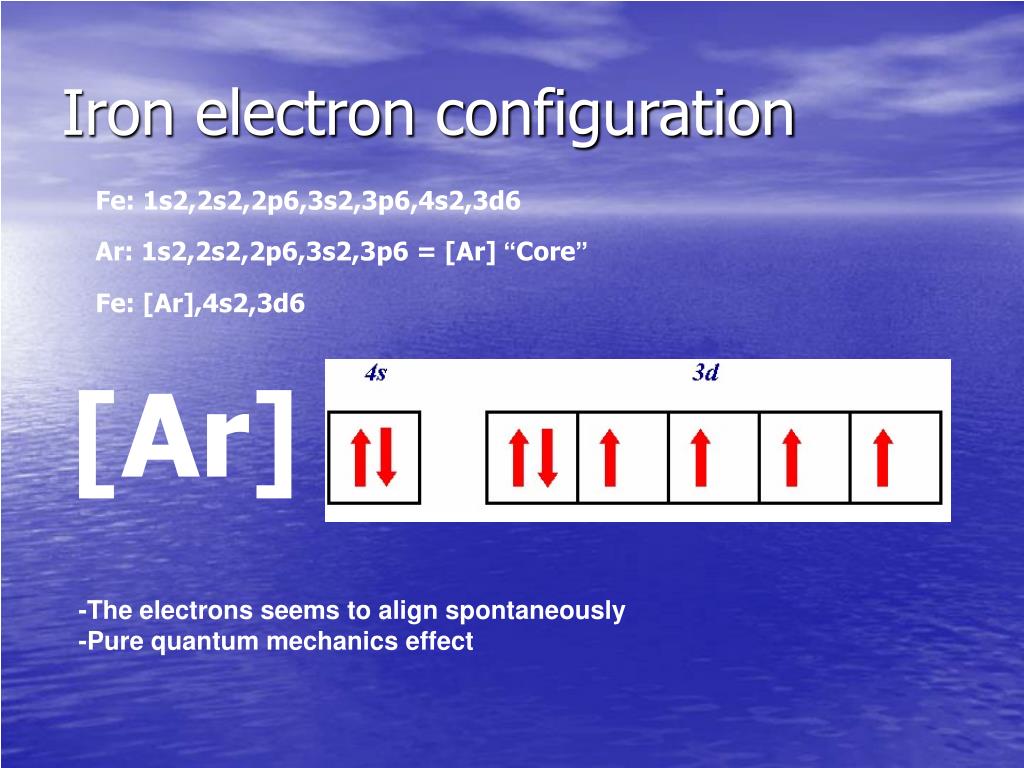
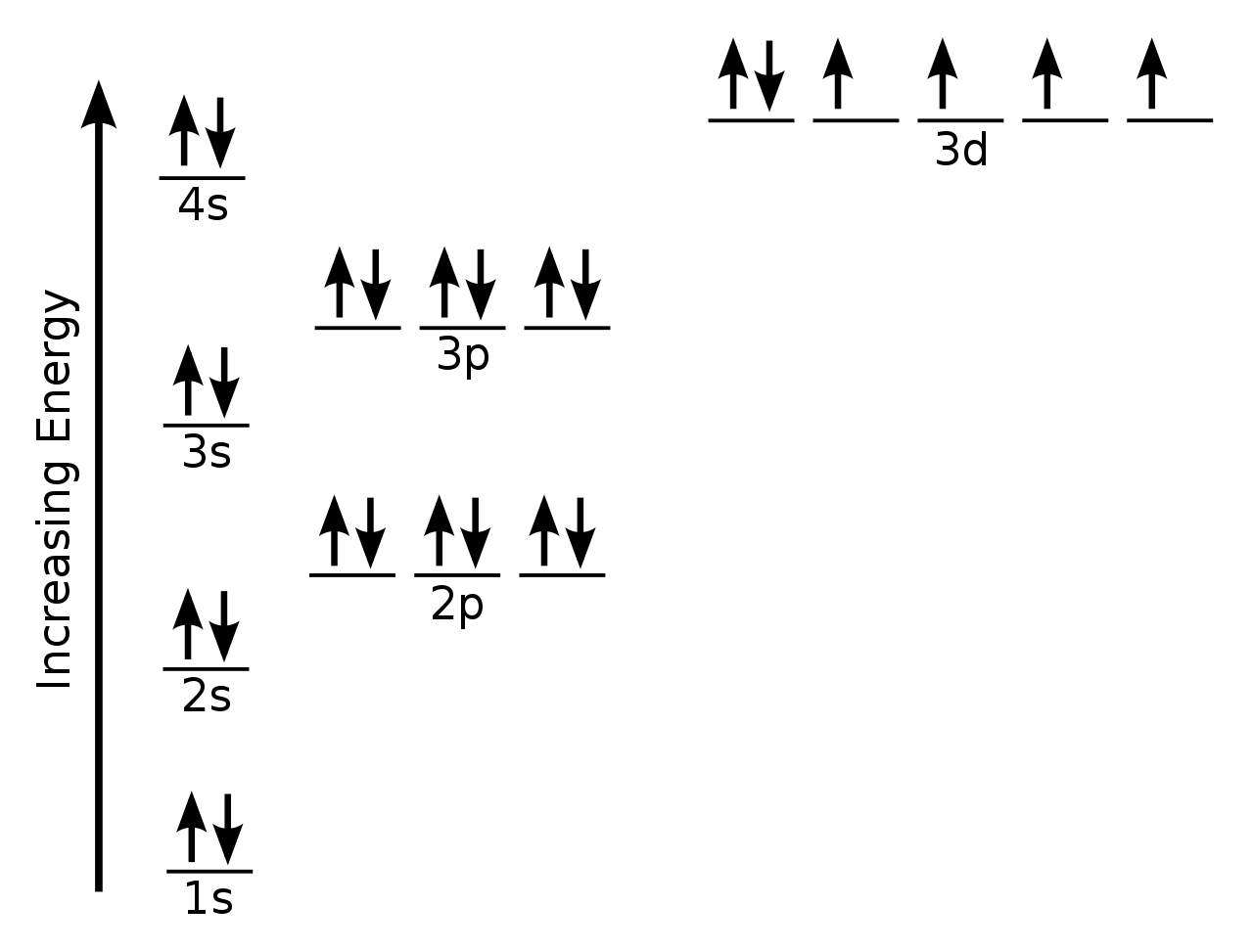
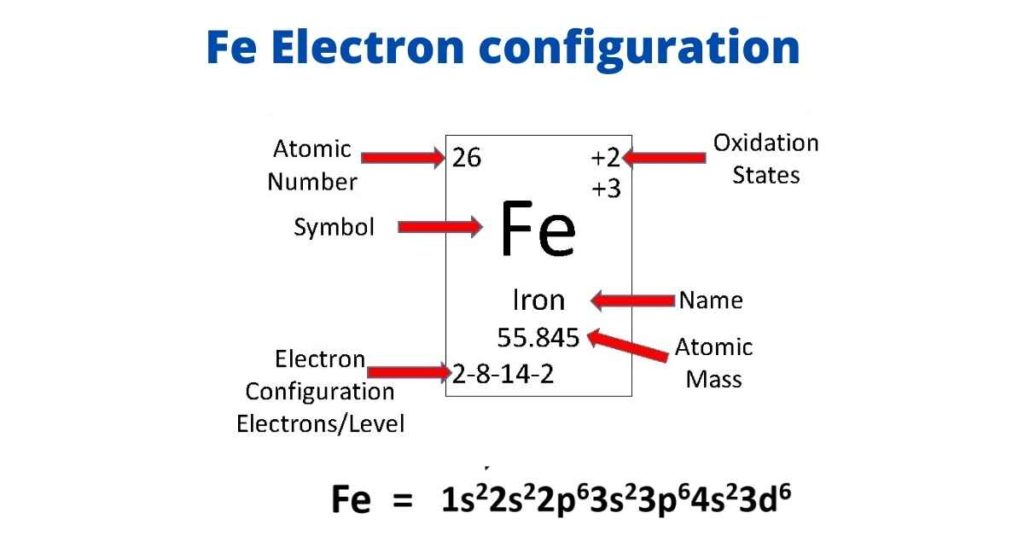
Iron Electron Configuration Long Form
Iron Electron Configuration Long Form - Iron is element 26 in the periodic table. These is known as ferromagnetic materials. Electron configuration of oxygen (o) [he] 2s 2 2p 4: 3d64s2 3 d 6 4 s 2. 1s 2 2s 2 2p 5: Determine whether the substance is paramagnetic or diamagnetic Iron have 2 valence electrons around the nucleus and the atomic number is 26.the distribution of electrons is as 2 electrons in 1s subshell, 2 electrons in 2s subshell, 6 electrons in 2p subshell, 2 electrons in 3s, 6 electrons in 3p 2 electrons in 4s and 6 electrons i. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Contact us join the conversation. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. The iron ion acquires a 2+ charge as a result. 1s 2 2s 2 2p 5: The peculiar crystalline structure and its electronic and electronic configuration make it naturally attractive to metals. Determine whether the substance is paramagnetic or diamagnetic Web electron configuration of carbon (c) [he] 2s 2 2p 2: In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Comprising 35% of the earth’s mass, iron is the main component of steel and the most used of all the metals. Web when we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. The iron. These is known as ferromagnetic materials. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Web electron configuration of carbon (c) [he] 2s 2 2p 2: The peculiar crystalline structure and its electronic and electronic configuration make it naturally attractive to metals. Binding energies of common chemical states: Web of the elements discussed in the video, iron is most like sc, but iron has 5 more electrons in the last subshell which is filled with electrons making its configuration end in 3d^6 rather than 3d^1 hope this helps! Relate electron configurations to element classifications in the periodic table. Contact us join the conversation. Iron is element 26 in. Web electronic configuration of iron is ar a r. Determine whether the substance is paramagnetic or diamagnetic Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time consuming and often not as practical as the spdf notation, especially for atoms with much longer configurations. Fe, fe2+, and fe3+ electron configuration notation. Electron. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Fe2+ f e 2 +: Web the complete electron configuration of iron is 1s2 2s 2 2p6 3s2 3p6 4s2 3d6. 3134 k (2861 °c, 5182 °f) density (near r.t.) 7.874 g/cm 3: Identify and. Ignore the core electrons and focus on the valence electrons only. Web when we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron has various types of allotropic forms in spite of not having a single crystalline structure. Fe2+ f e 2 +: 3134 k (2861 °c, 5182 °f) density (near. Web in order to write the electron configuration for iron (fe) we first need to know the number of electrons for the fe atom (there are 26 electrons). For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Iron is element 26 in the periodic. Web this video help for you.how to write the electron configuration iron (fe).#ironelectronicconfiguration#careervalleyinstitute#electronicconfiguration Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time consuming and often not as practical as the spdf notation, especially for atoms with much longer configurations. 3134 k (2861 °c, 5182 °f) density (near r.t.) 7.874 g/cm 3:. This page shows the electron configurations of the neutral gaseous atoms in their ground states. There is one unpaired electron. Binding energies of common chemical states: For cl atoms, the electron configuration is 3s 2 3p 5. Comprising 35% of the earth’s mass, iron is the main component of steel and the most used of all the metals. Web electron configuration of carbon (c) [he] 2s 2 2p 2: 1s 2 2s 2 2p 5: Web in order to write the electron configuration for iron (fe) we first need to know the number of electrons for the fe atom (there are 26 electrons). The iron ion acquires a 2+ charge as a result. 1s 2 2s 2 2p 3: Binding energies of common chemical states: Ignore the core electrons and focus on the valence electrons only. Web electron configuration 3d 6 4s 2: Fe, fe2+, and fe3+ electron configuration notation. Electron configuration of oxygen (o) [he] 2s 2 2p 4: Web when we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Comprising 35% of the earth’s mass, iron is the main component of steel and the most used of all the metals. There is one unpaired electron. Identify and explain exceptions to predicted electron configurations for atoms and ions. Contact us join the conversation. Electron configuration of fluorine (f) [he] 2s 2 2p 5: 3134 k (2861 °c, 5182 °f) density (near r.t.) 7.874 g/cm 3: Determine whether the substance is paramagnetic or diamagnetic Electron configuration of nitrogen (n) [he] 2s 2 2p 3:PPT PowerPoint Presentation, free download ID855242
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB)
Electron Configuration of Transition Metals Iron Fe Cobalt Co and
List of Electron Configurations of Elements
Iron electronic configuration How to Write Iron electronic
Железо это... Что такое Железо?
Day 3 Iron An Element A Day
Electron Configuration for Iron (Fe) What's Insight
Flashcard of Iron with atomic mass Royalty Free Vector Image
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions
Related Post: