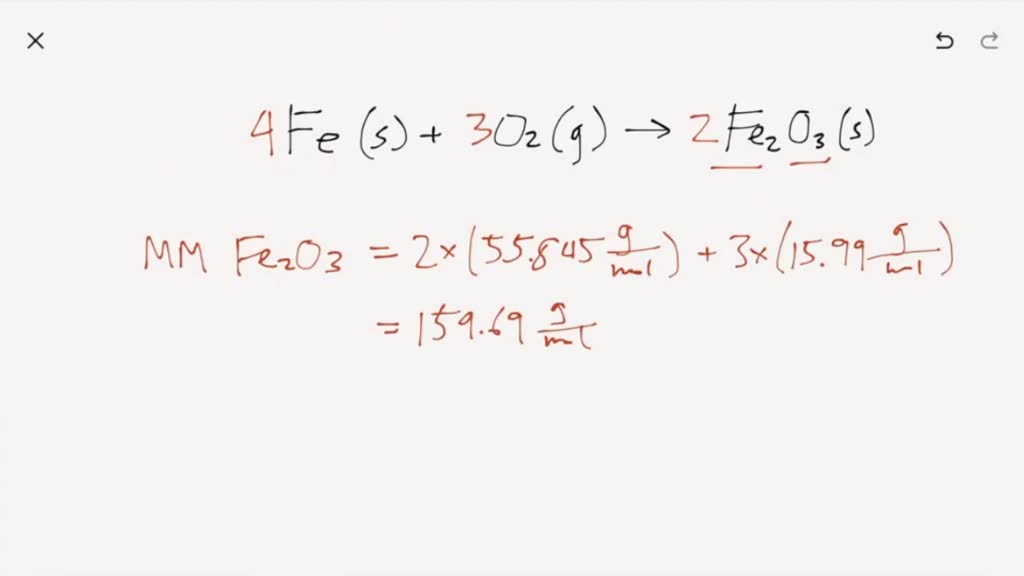Iron And Oxygen Gas React To Form Iron Iii Oxide
Iron And Oxygen Gas React To Form Iron Iii Oxide - Write the balanced chemical equation for this reaction. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. How many moles of iron are needed to make 5.0 moles of iron (iii) oxide? Write the skeleton equation for the reaction. Diatomic elements are always diatomic (written with. Write the balanced equation for. 4 f e ( s) + 3 o 2 ( g) → 2 f e 2 o 3 ( s) if 6.4 moles of fe react with excess o 2, how many moles of f e 2 o 3 can be formed?. Using mulliken's formula, calculate a value for the electronegativity of. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. Web iron and oxygen react to form iron(iii) oxide according to the chemical equation: Web when iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. How many moles of iron are needed to make 5.0 moles of iron (iii) oxide? Web iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. Web iron and oxygen gas react in a combination reaction to. 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0 moles of fe are reacted with 6.0 moles of o2 what would be. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. Write the balanced equation for. Web iron reacts with oxygen to produce iron (iii) oxide. Iron +. Web write the balanced equation for the rusting of iron in which iron reacts with oxygen to form iron (iii) oxide. Iron + water + oxygen → hydrated. Web the iron reacts with water and oxygen to form hydrated iron (iii) oxide, which we see as rust. Web iron reacts with oxygen gas to form iron (iii) oxide according to. 4+fezz 4 fe (s) + 3 02 (g) →feo; Web reaction of iron (iii) oxide ( fe 2o 3) with carbon monoxide ( co) to form iron ( fe) and carbon dioxide ( co 2 ), is a redox reaction as both oxidation of carbon monoxide and. How many moles of iron are needed to make 5.0 moles of iron. Using mulliken's formula, calculate a value for the electronegativity of. Here is the word equation for the reaction: Web iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. Balanced equation for the oxidation of iron sulfide. Web when iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. How many moles of iron are needed to make 5.0 moles of iron (iii) oxide? Web reaction of iron (iii) oxide ( fe 2o 3) with carbon monoxide ( co) to form iron ( fe) and carbon dioxide ( co 2 ), is a redox reaction as both oxidation of carbon monoxide and. Web iron and oxygen combine to form. Balanced equation for the oxidation of iron sulfide. Write the balanced chemical equation for this reaction. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. 4 f e ( s) + 3 o 2 ( g) → 2 f e 2 o 3 ( s) if 6.4 moles of fe react with excess o. Web when iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. Web iron + water + oxygen → hydrated iron. Web iron and oxygen react to form iron(iii) oxide according to the chemical equation: Iron (iii) sulfide (fe₂s₃) reacts with oxygen to produce iron (iii) oxide (fe₂o₃) and sulfur dioxide (so₂). Web iron and oxygen. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. Web when iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. Write the balanced chemical equation for this reaction. Web solid iron and gaseous chlorine react to produce a solid iron (iii) chloride. Web iron. Web iron and oxygen react to form iron(iii) oxide according to the chemical equation: 4 f e ( s) + 3 o 2 ( g) → 2 f e 2 o 3 ( s) if 6.4 moles of fe react with excess o 2, how many moles of f e 2 o 3 can be formed?. Web reaction of iron. Diatomic elements are always diatomic (written with. Here is the word equation for the reaction: Web iron and oxygen combine to form iron oxide, or rust. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. Balanced equation for the oxidation of iron sulfide. Web write the balanced equation for the rusting of iron in which iron reacts with oxygen to form iron (iii) oxide. Web iron can react with oxygen to. Web iron and oxygen react to form iron(iii) oxide according to the chemical equation: Web iron reacts with oxygen to produce iron (iii) oxide. Web iron and oxygen gas react in a combination reaction to form iron (iii) oxide. Write the balanced equation for. How many moles of iron are needed to make 5.0 moles of iron (iii) oxide? Web reaction of iron (iii) oxide ( fe 2o 3) with carbon monoxide ( co) to form iron ( fe) and carbon dioxide ( co 2 ), is a redox reaction as both oxidation of carbon monoxide and. Web iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. Write the balanced chemical equation for this reaction. 4 f e ( s) + 3 o 2 ( g) → 2 f e 2 o 3 ( s) if 6.4 moles of fe react with excess o 2, how many moles of f e 2 o 3 can be formed?. Write the skeleton equation for the reaction. Iron + water + oxygen → hydrated. 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0 moles of fe are reacted with 6.0 moles of o2 what would be. Web this reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide.Solved 6. Iron reacts with oxygen gas to form iron (III)
How to Write the Formula for Iron (III) Oxide YouTube
PPT Unit Chemical Reactions PowerPoint Presentation, free download
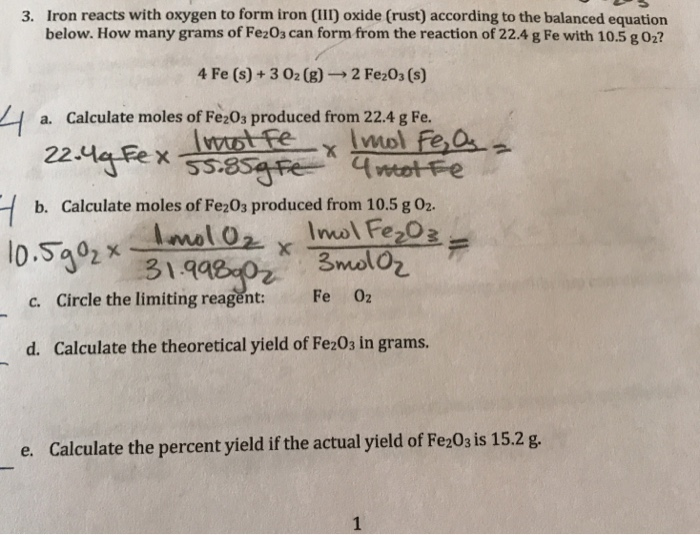
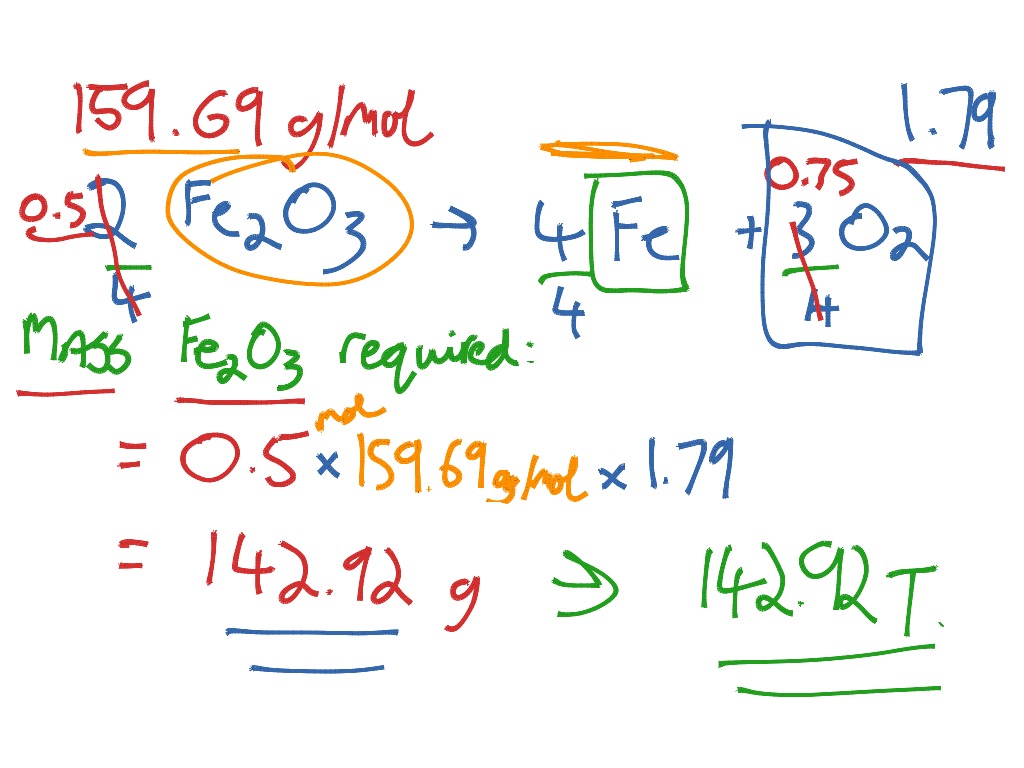
Solved 3. Iron reacts with oxygen to form iron (III) oxide
of Iron(III)oxide Science, Chemistry, Stoichiometry
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a
[Solved] Solid Iron (III) reacts with oxygen gas to form iron (III
Balance Fe + O2 = Fe2O4 (Iron and Oxygen yields Iron (II) Oxide) YouTube
when iron rusts, solid iron reacts with gaseous oxygen to form solid
Related Post: