In General What Determines Whether Atoms Will Form Chemical Bonds
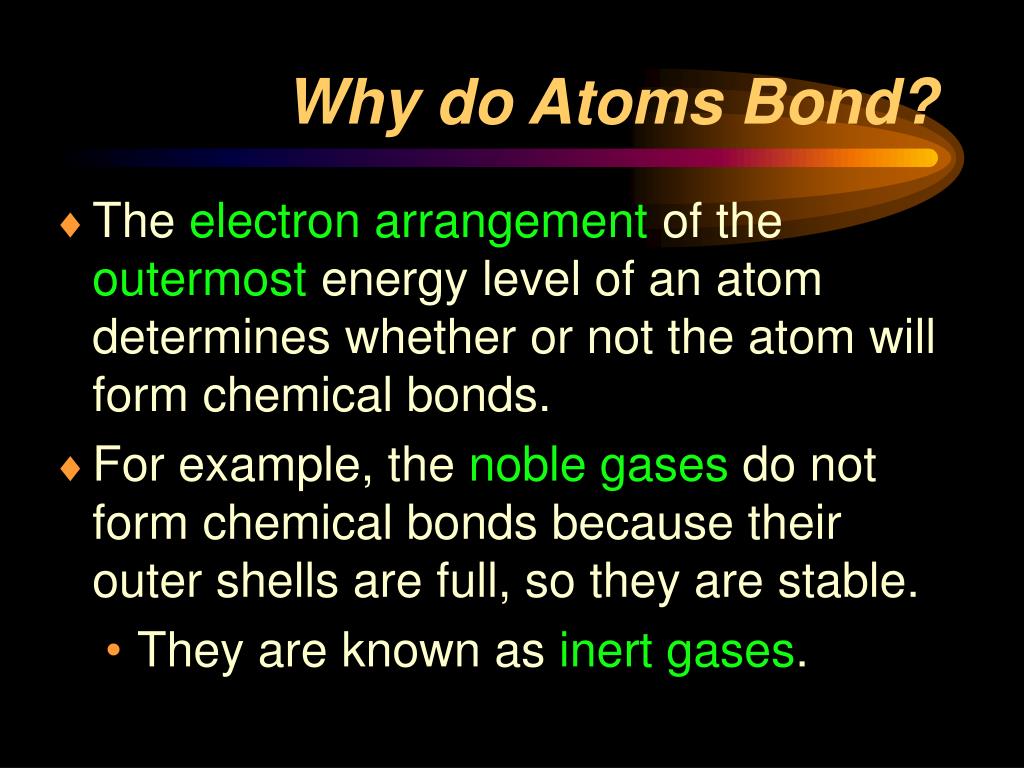
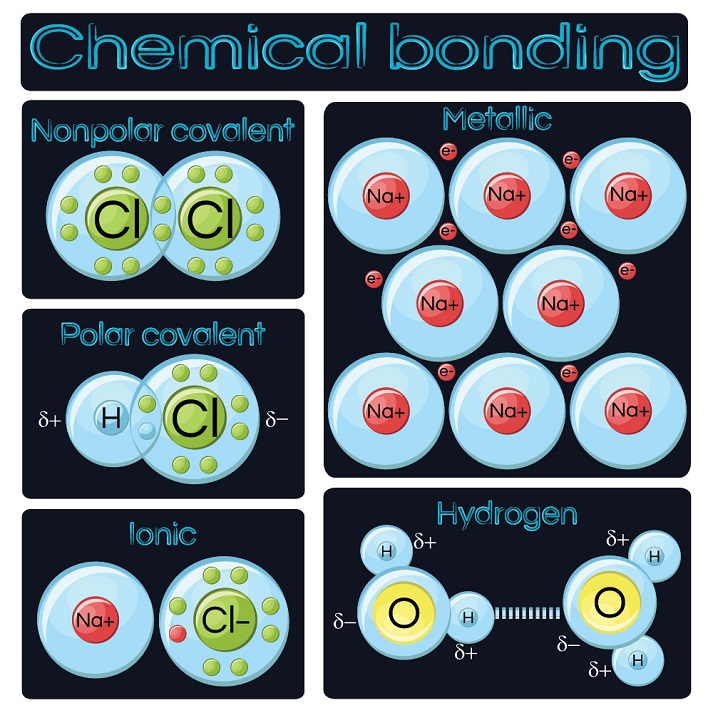
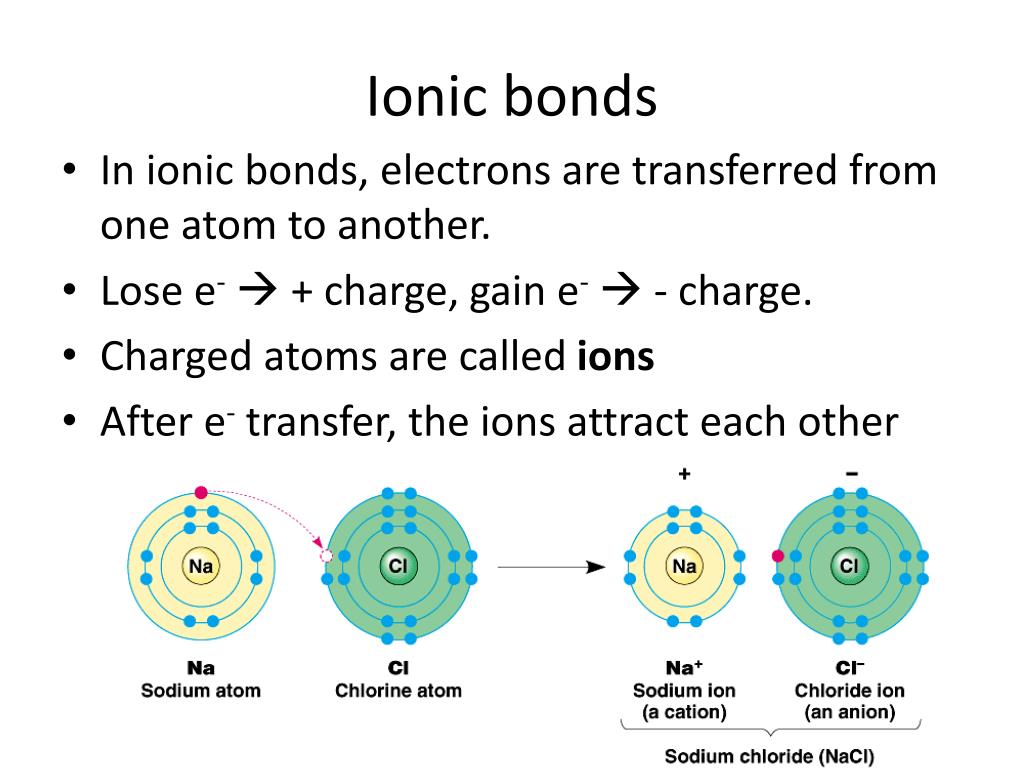
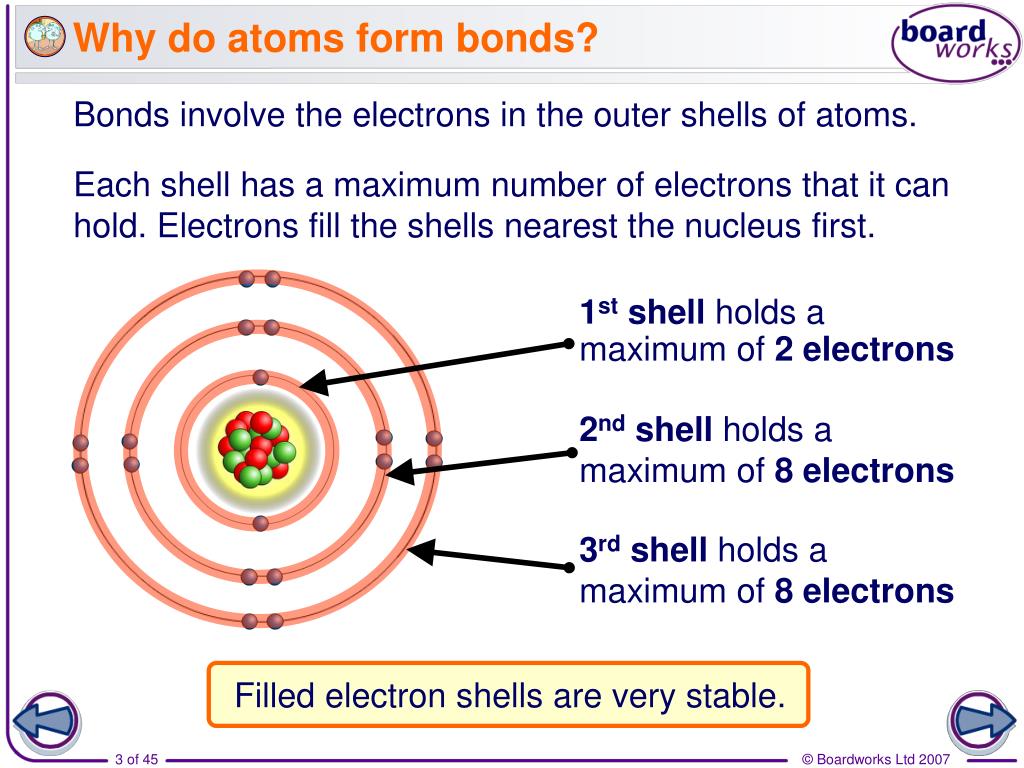
In General What Determines Whether Atoms Will Form Chemical Bonds - Web what determines whether an atom will form a chemical bond with another atom? Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. > since the valence electrons are the outermost. Web polar covalent bonds. If the potential energy will be lowered by the bond forming what kind of bond is formed by h and i if the. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The potential energy of two separate hydrogen atoms (right) decreases as. So this requires us to be able to make various chemical bonds between the. Web bond dissociation energy basically being the energy input required to break a chemical bond. Web in general, what determines whether atoms will form chemical bonds? The two most basic types of bonds are characterized as either ionic or. Web two or more atoms are linked together by chemical bonds. Web what determines whether atoms will form chemical bonds? The bond is caused by the. So this requires us to be able to make various chemical bonds between the. Web what determines whether atoms will form chemical bonds? The substances that go into a chemical reaction are called the reactants , and the. The number of electrons in its outermost shell. A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds the atoms together. When doing so, the valence. So this requires us to be able to make various chemical bonds between the. The number of electrons in its outermost shell. A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds the atoms together. Web what determines whether atoms will form chemical bonds? A bond in which the electronegativity difference. The two most basic types of bonds are characterized as either ionic or. The number of electrons in its outermost shell. So this requires us to be able to make various chemical bonds between the. The potential energy of two separate hydrogen atoms (right) decreases as. Web chemical reactions occur when chemical bonds between atoms are formed or broken. Atoms react to form bonds when the number of electron in the outermost shell is not maximum. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. Some atoms become more stable by gaining or losing an entire electron (or several electrons). The. Web there are many types of chemical bonds and forces that bind molecules together. It depends on if one atom needs another electron why are all of the elements in group 8a relatively. If the potential energy will be lowered by the bond forming what kind of bond is formed by h and i if the. The substances that go. Nature favors arrangements in which potential energy minimized by bonding with each other. The substances that go into a chemical reaction are called the reactants , and the. Some atoms become more stable by gaining or losing an entire electron (or several electrons). The number of electrons in its outermost shell. Web polar covalent bonds. Web two or more atoms are linked together by chemical bonds. Web polar covalent bonds. The electron arrangement of the outer energy level of an atom determines whether or not it will form. > since the valence electrons are the outermost. A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds. Web what determines whether an atom will form a chemical bond with another atom? Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. What determines whether atoms will form chemical bonds is to maximize the stability. When doing so, the valence. Web chemical bonds. Is determined by the distance at which the lowest potential energy is achieved. The number of electrons in its outermost shell. What determines whether atoms will form chemical bonds is to maximize the stability. The stability of the atom's nucleus the number of protons in the atom's nucleus the state of. Web in general, what determines whether atoms will form. The substances that go into a chemical reaction are called the reactants , and the. Electron gain or loss can give an atom a filled outermost. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond is caused by the. Web there are many types of chemical bonds and forces that bind molecules together. When they do so, atoms form ions, or charged particles. So this requires us to be able to make various chemical bonds between the. The number of electrons in its outermost shell. When doing so, the valence. Web in general, what determines whether atoms will form chemical bonds? What determines whether atoms will form chemical bonds is to maximize the stability. > since the valence electrons are the outermost. Nature favors arrangements in which potential energy minimized by bonding with each other. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. The two most basic types of bonds are characterized as either ionic or. Web bond dissociation energy basically being the energy input required to break a chemical bond. Web a chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. Some atoms become more stable by gaining or losing an entire electron (or several electrons). A chemical bond is a mutual electrical attraction between the nuclei in valence, electrons of different atoms that binds the atoms together. Web what determines whether an atom will form a chemical bond with another atom?PPT Types of Bonds PowerPoint Presentation, free download ID1996079
Chemical Bonds Definition, Types, and Examples
PPT Chapter 7 Atoms & Bonding PowerPoint Presentation, free download
Metallic Chemical Bonds Educational Resources K12 Learning, Chemistry
PPT Atomic structure and chemical bonding PowerPoint Presentation
covalent bond Definition, Properties, Examples, & Facts Britannica
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
The top panel in this figure shows two hydrogen atoms sharing two
Molecules Compounds Bonds « KaiserScience
Related Post: