Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas.
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas. - Web nitrogen and hydrogen gas react to form ammonia gas as follows: We must remember that nitrogen and hydrogen are both. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. Web nitrogen and hydrogen gas react to form ammonia gas as follows: Web the volume of nitrogen (n₂) gas = 7.7 ml. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of nitrogen is required to completely react with 800.0. Web the volume of ammonia that would be produced by this reaction if 6.9 m3 of hydrogen were consumed is 4,600.064cm³. What volume of ammonia would be produced by this reaction if 6.9 m3 of nitrogen were consumed? What volume of ammonia would be produced by this reaction if 5,4 ml. Hydrogen gas and nitrogen gas react to form ammonia gas. Web nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. N2 ( g)+3h2 ( g)→2nh3 ( g) if all the n2 and h2 are consumed, what volume of nh3, at the. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; 2 moles. 1 ml = 0.001 l. Web nitrogen and hydrogen gas react to form ammonia gas as follows: Hydrogen gas and nitrogen gas react to form ammonia gas. N2 ( g)+3h2 ( g)→2nh3 ( g) if all the n2 and h2 are consumed, what volume of nh3, at the. This reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web reaction of hydrogen and nitrogen to form ammonia. Web nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Thus, 7.7 ml = 0.0077 l. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Thus, 7.7 ml = 0.0077 l. We must remember that nitrogen and hydrogen are both. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: N2 (g) + 3h2 (g) → 2nh3 (g) at a certain temperature and pressure, 1.2 l of nitrogen gas reacts with 3.6 l. 1. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 (. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: Hydrogen gas, h2, reacts with nitrogen gas, n2, to form ammonia gas, nh3, according to the equation. Also, be sure your answer has a. Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: Web nitrogen and hydrogen gas react to form ammonia gas as follows: 1 ml = 0.001 l. It would be interesting to know how the molecules do interact. Web nitrogen and hydrogen gases react to form ammonia gas via. N2 (g) + 3h2 (g) → 2nh3 (g) at a certain temperature and pressure, 1.2 l of nitrogen gas reacts with 3.6 l. N2(g) + 3h2(g) → 2nh3(g) at a certain temperature and pressure, 1.2 l of nitrogen gas. 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. What volume of ammonia would be produced. Web may 13, 2014. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of nitrogen is required to completely react with 800.0. Web hydrogen then does not undergo a chemical reaction with ammonia but the interaction is physical. It would be interesting to know how the molecules do interact. Hydrogen gas and nitrogen. Hydrogen gas, h2, reacts with nitrogen gas, n2, to form ammonia gas, nh3, according to the equation. This reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Also, be sure your answer has a unit symbol, and is rounded. What volume of. N2 (g) + 3h2 (g) → 2nh3 (g) at a certain temperature and pressure, 1.2 l of nitrogen gas reacts with 3.6 l. Web the volume of nitrogen (n₂) gas = 7.7 ml. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2 reacts with. Web the volume of ammonia that would be. 1 ml = 0.001 l. Thus, 7.7 ml = 0.0077 l. N2 (g) + 3h2 (g) → 2nh3 (g) at a certain temperature and pressure, 1.2 l of nitrogen gas reacts with 3.6 l. N + h → n h 3. Web hydrogen then does not undergo a chemical reaction with ammonia but the interaction is physical. Web nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Web nitrogen and hydrogen gas react to form ammonia gas as follows: Web the volume of nitrogen (n₂) gas = 7.7 ml. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of nitrogen is required to completely react with 800.0. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web to calculate the number of moles of hydrogen needed, we can use the stoichiometric ratio from the balanced chemical equation: 2 moles n2 * (3 moles h2 / 1 mole n2) = 6 moles. Hydrogen gas, h2, reacts with nitrogen gas, n2, to form ammonia gas, nh3, according to the equation. What volume of ammonia would be produced by this reaction if 6.9 m3 of nitrogen were consumed? This reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Web reaction of hydrogen and nitrogen to form ammonia. The reaction between the hydrogen gas. What volume of ammonia would be produced by this reaction if 5,4 ml. Hydrogen gas and nitrogen gas react to form ammonia gas.⚗️Question 6 (1 point) Hydrogen gas and nitrogen gas can react to form
⚗️Hydrogen gas and nitrogen gas can react to form ammonia according to
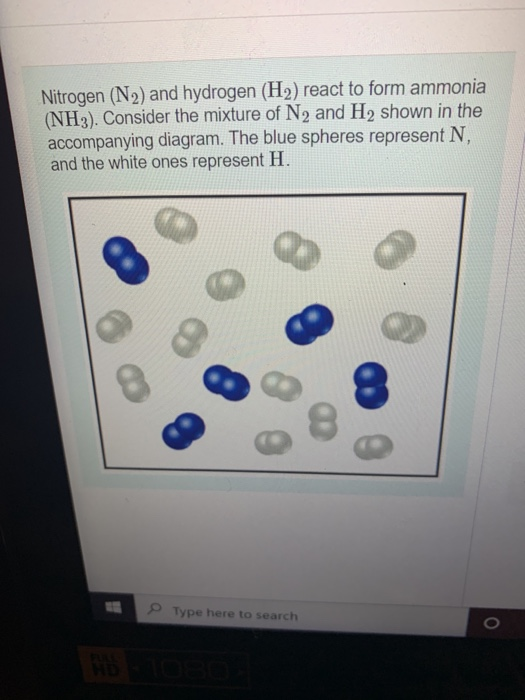
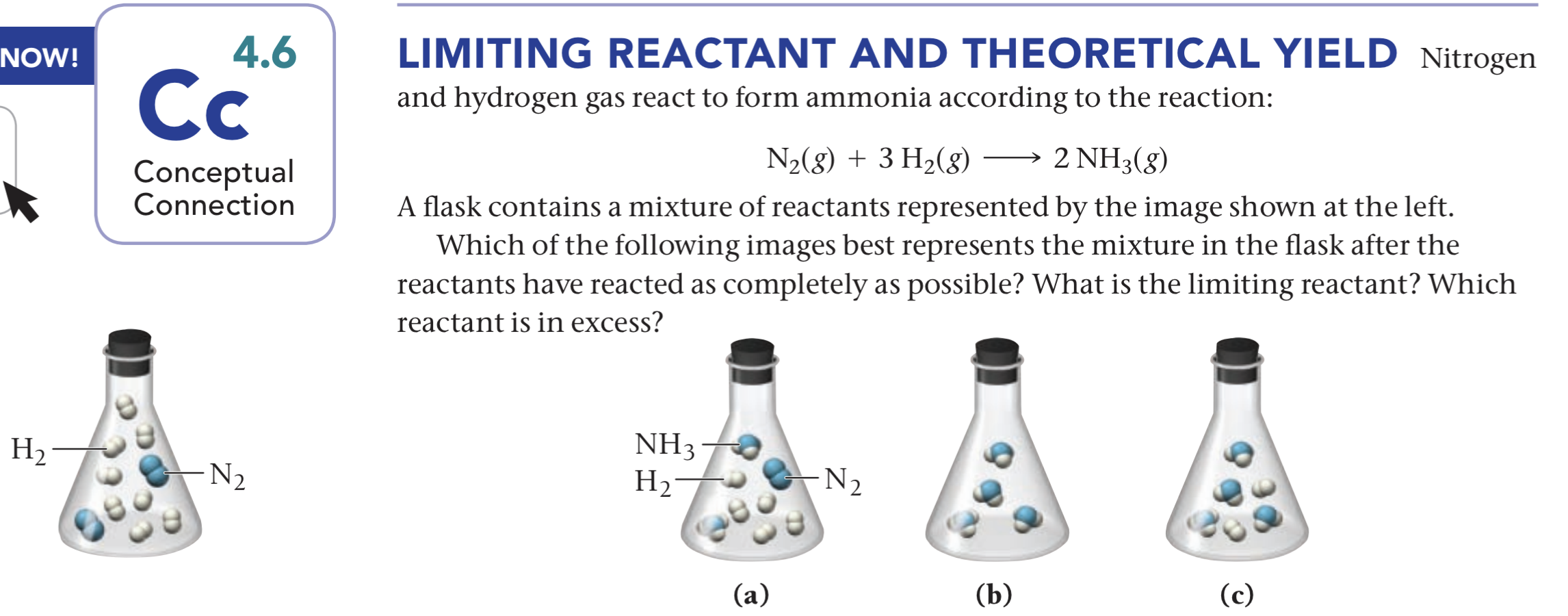
Solved Nitrogen (N2) and hydrogen (H2) react to form ammonia
Nitrogen Gas Reacts With Hydrogen Gas To Form Ammonia Printable Form
1. The cartoon below represents the reaction of nitrogen gas (N2) with
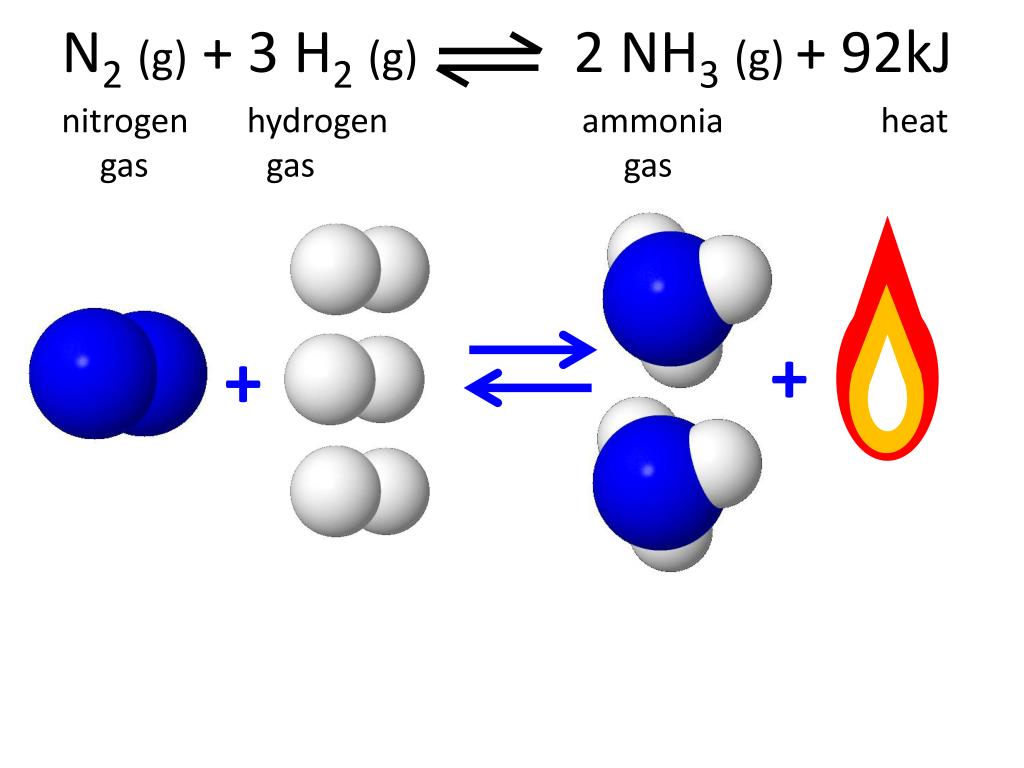
Hydrogen Gas Combines with Nitrogen to form ammonia?
[Solved] Hydrogen gas and nitrogen gas can react to form ammonia
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
PPT Reaction Rates PowerPoint Presentation, free download ID6516140
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
Related Post: