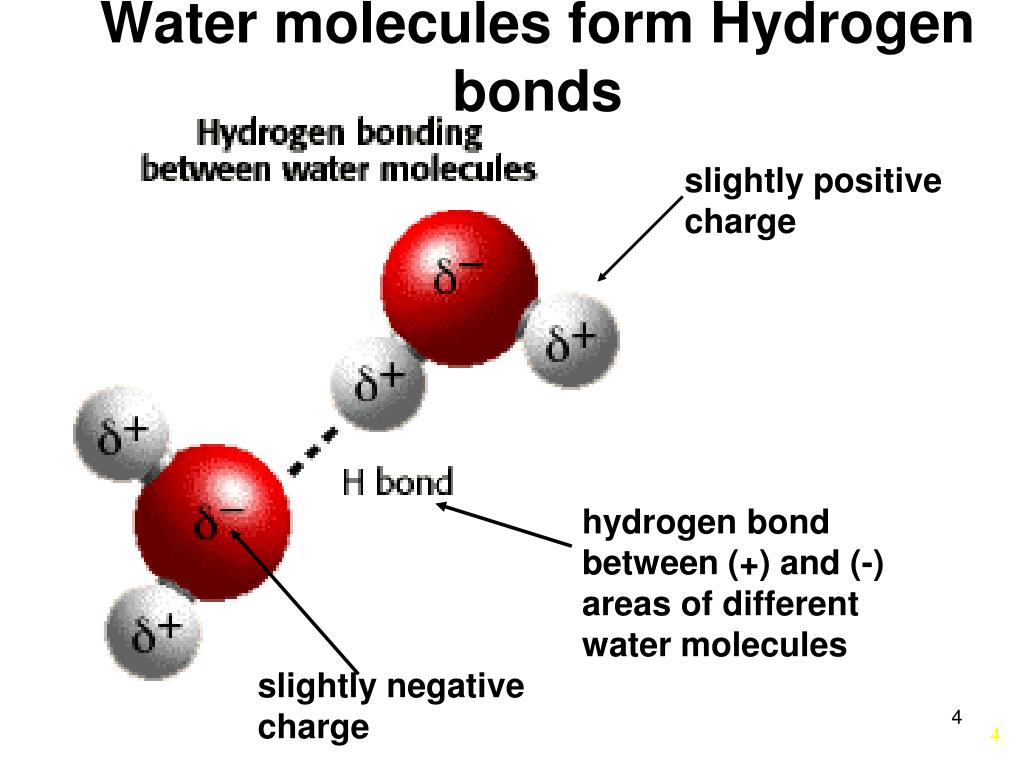
Hydrogen Bonds Form Between Adjacent Water Molecules Because The
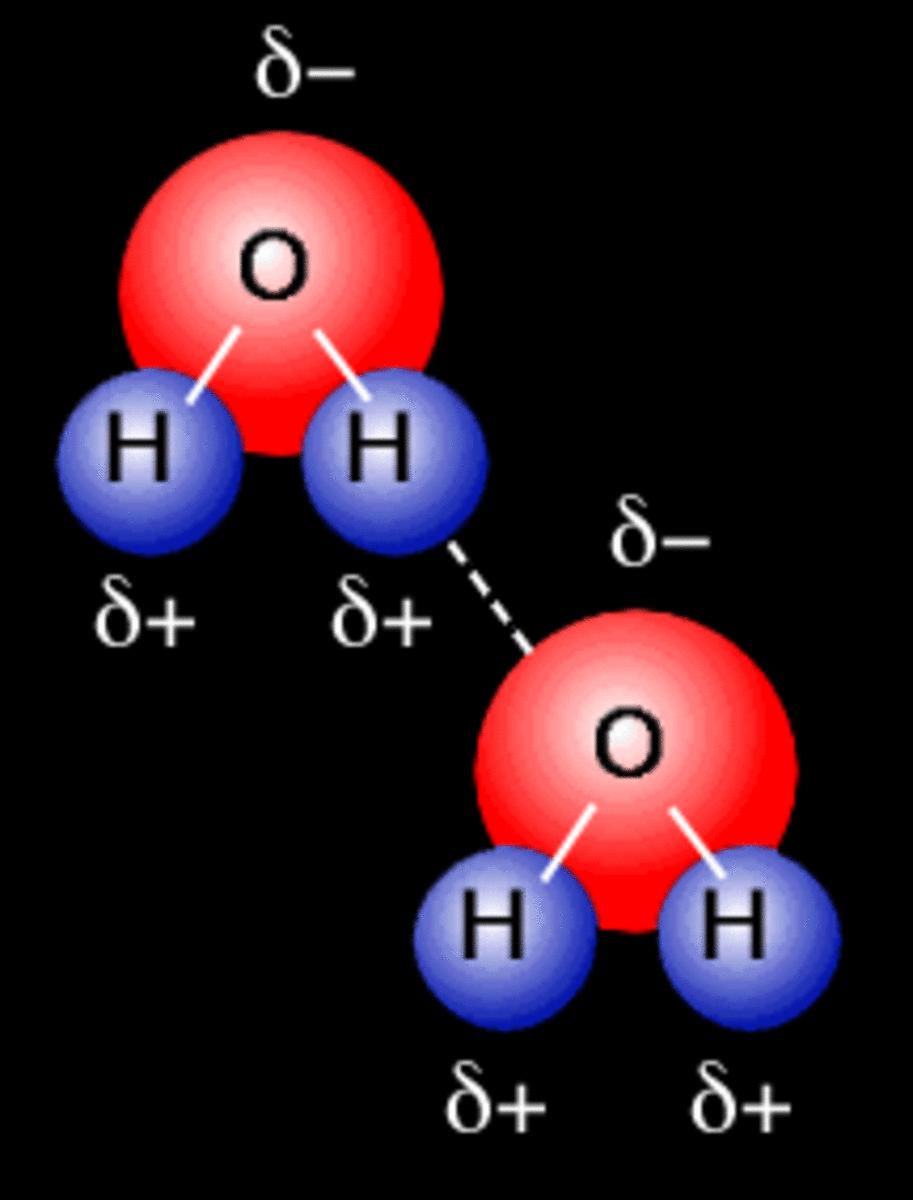
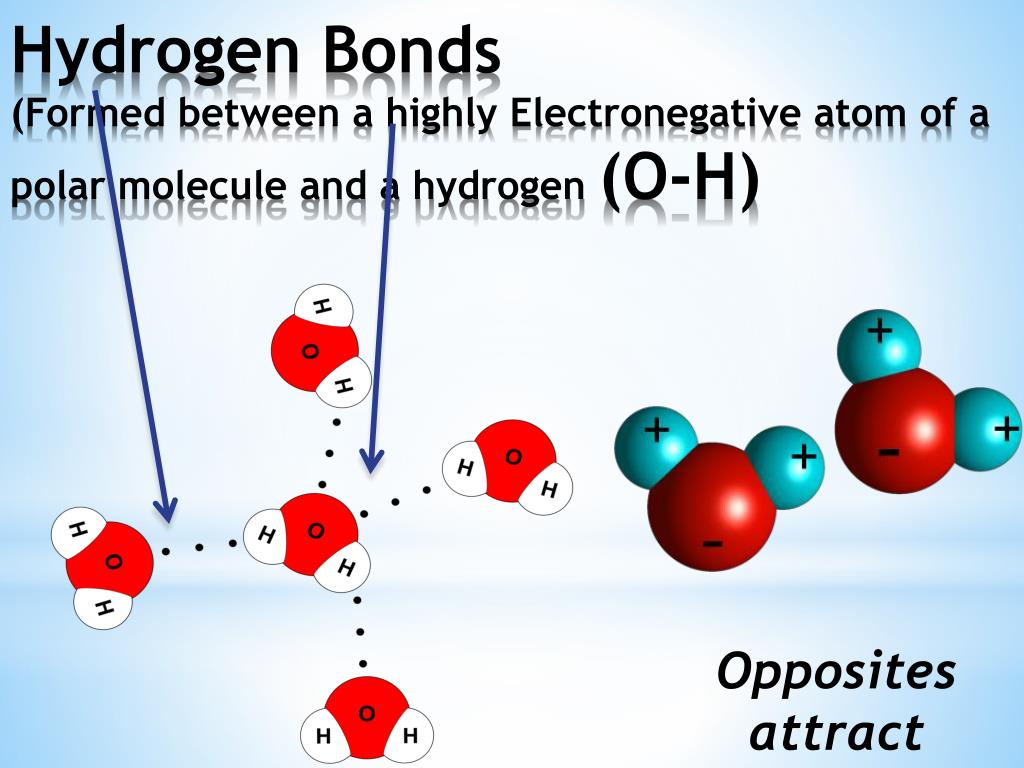
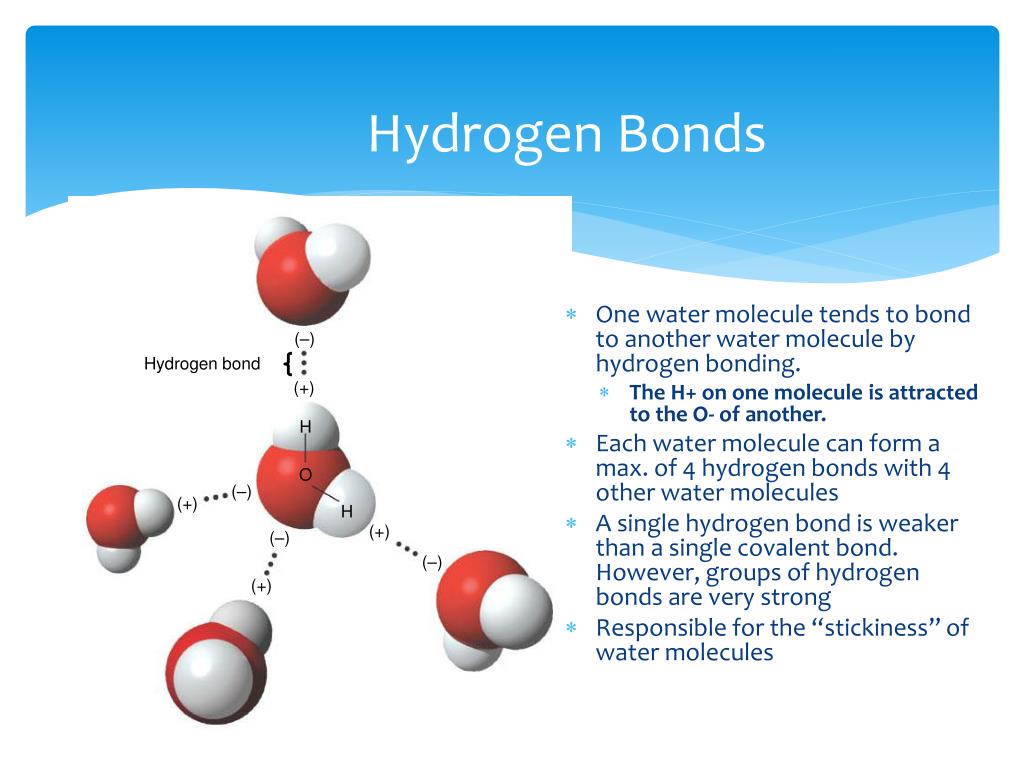
Hydrogen Bonds Form Between Adjacent Water Molecules Because The - Hydrogen bonding hydrogen bonds form between adjacent water molecules due to charge differences between oxygen and. Water has an amazing ability to adhere (stick) to itself and to other substances. Hydrogen bonds make water sticky. Web because of this attraction, bonds form between hydrogen and oxygen atoms of adjacent water molecules, as demonstrated in figure \(\pageindex{4}\). The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. Hydrogen bonds are strong intermolecular interactions that can form between neighboring molecules. Web instead, each hydrogen atom is 101 pm from one oxygen and 174 pm from the other. Hydrogen bonds generally only form between molecules that. Hydrogen bonding between different parts of the same chain (intramolecular. Web a hydrogen bond is usually indicated by a dotted line between the hydrogen atom attached to o, n, or f (the hydrogen bond donor) and the atom that has the lone pair of. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. Ad browse & discover thousands of science book titles, for less. Web hydrogen bonding between adjacent polymer chains. Web hydrogen bonding in water. Web instead, each hydrogen atom is 101 pm from one oxygen and 174 pm from the other. Hydrogen bonds make water sticky. Charged hydrogen end of one water molecule attracts the. In contrast, each oxygen atom is bonded to two h atoms at the shorter. Two with the hydrogen atoms and two with the with. Web water has the property of adhesion because: Web because of this attraction, bonds form between hydrogen and oxygen atoms of adjacent water molecules, as demonstrated in figure \(\pageindex{4}\). However, because they are exposed to air on one side, they will have fewer neighboring water molecules to. Hydrogen bonds form. Hydrogen bonding between different parts of the same chain (intramolecular. Web water (h 2 o): The bond is between the hydrogen of one water molecule and the oxygen atoms of another. Hydrogen bonds are strong intermolecular interactions that can form between neighboring molecules. Water has an amazing ability to adhere (stick) to itself and to other substances. Web a hydrogen bond is usually indicated by a dotted line between the hydrogen atom attached to o, n, or f (the hydrogen bond donor) and the atom that has the lone pair of. However, because they are exposed to air on one side, they will have fewer neighboring water molecules to. Hydrogen bonds make water sticky. This type of. Web water (h 2 o): This results in water's ability to stick to itself. This type of bond always. Water molecules forming hydrogen bonds with one another. The bond is between the hydrogen of one water molecule and the oxygen atoms of another. This results in water's ability to stick to itself. Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); Hydrogen bonding between different parts of the same chain (intramolecular. Water molecules forming hydrogen bonds with one another. Water has an amazing ability to adhere (stick) to itself and to other substances. Water molecules forming hydrogen bonds with one another. Hydrogen bonds form between adjacent water molecules, creating a cohesive force. In contrast, each oxygen atom is bonded to two h atoms at the shorter. Charged hydrogen end of one water molecule attracts the. Hydrogen bonds are strong intermolecular interactions that can form between neighboring molecules. Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); Web the hydrogen bonding in water is between one of the hydrogen atoms of one molecule and the oxygen atom of an adjacent water molecule. Hydrogen bonds make water sticky. Hydrogen bonds form between adjacent water molecules.' hydrogen bonds. Web instead, each hydrogen atom is 101 pm from one oxygen and 174 pm from the other. Hydrogen bonding between different parts of the same chain (intramolecular. Water molecules forming hydrogen bonds with one another. The bond is between the hydrogen of one water molecule and the oxygen atoms of another. Web because of this attraction, bonds form between hydrogen. Web water (h 2 o): Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Water molecules forming hydrogen bonds with one another. The bond is between the hydrogen of one water molecule and the oxygen atoms of another. Hydrogen bonding between different parts of the same chain (intramolecular. However, because they are exposed to air on one side, they will have fewer neighboring water molecules to. Web hydrogen bonds form between adjacent water molecules because the _______________ positively. This results in water's ability to stick to itself. Oxygen is highly electronegative, which creates a partial negative charge on one end of the. Web the hydrogen bonding in water is between one of the hydrogen atoms of one molecule and the oxygen atom of an adjacent water molecule. Web because of this attraction, bonds form between hydrogen and oxygen atoms of adjacent water molecules, as demonstrated in figure \(\pageindex{4}\). Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web instead, each hydrogen atom is 101 pm from one oxygen and 174 pm from the other. Hydrogen bonds generally only form between molecules that. In contrast, each oxygen atom is bonded to two h atoms at the shorter. Web hydrogen bonding between adjacent polymer chains (intermolecular bonding); Web a hydrogen bond is usually indicated by a dotted line between the hydrogen atom attached to o, n, or f (the hydrogen bond donor) and the atom that has the lone pair of. Web water has the property of adhesion because: Ad browse & discover thousands of science book titles, for less. Hydrogen bonds form between adjacent water molecules.' hydrogen bonds form between water molecules and hydrophilic.Primary and Secondary Bonds Owlcation
Diagram Of Water Molecule
PPT Properties of Water PowerPoint Presentation, free download ID
PPT Properties of Water PowerPoint Presentation, free download ID
Properties of Water Presentation Biology
Water
Soap and Water Interaction
PPT Water Chemistry & Properties of Water PowerPoint Presentation
Hydrogen Bonds — Overview & Examples Expii
Water ‹ OpenCurriculum
Related Post:

.PNG)