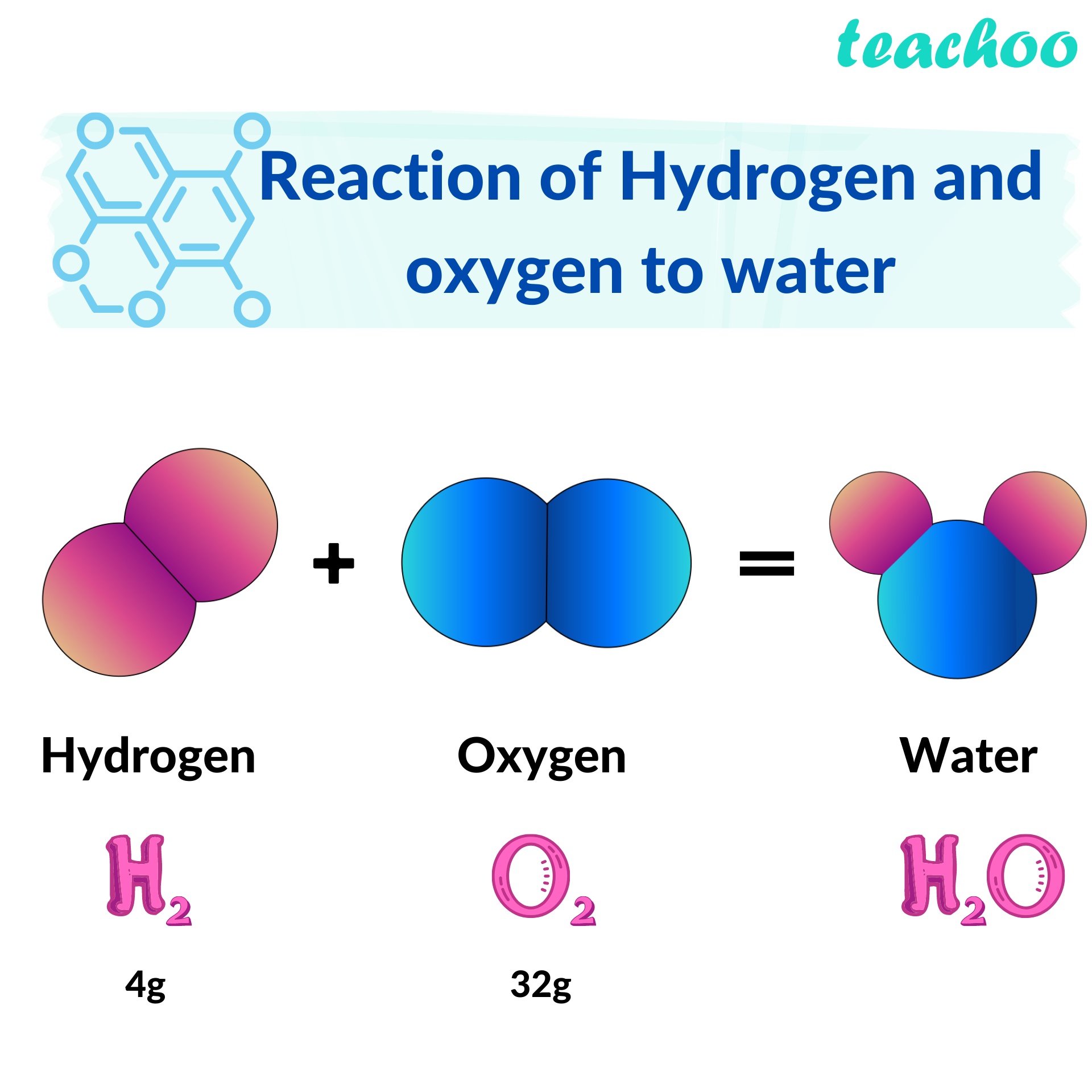
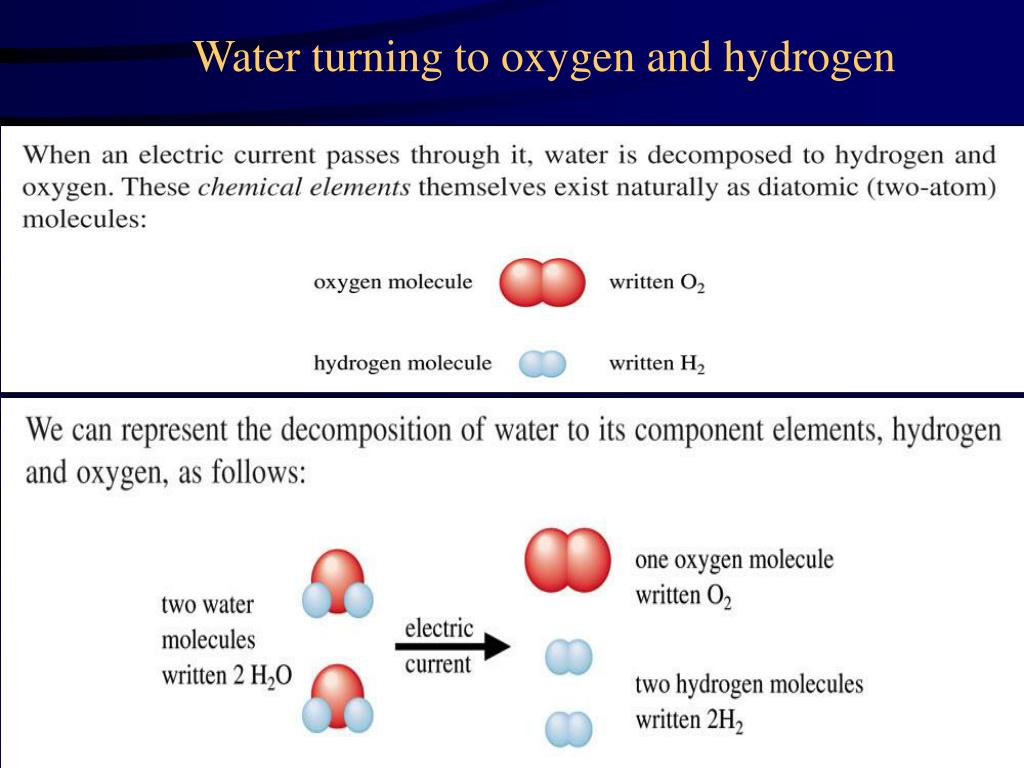
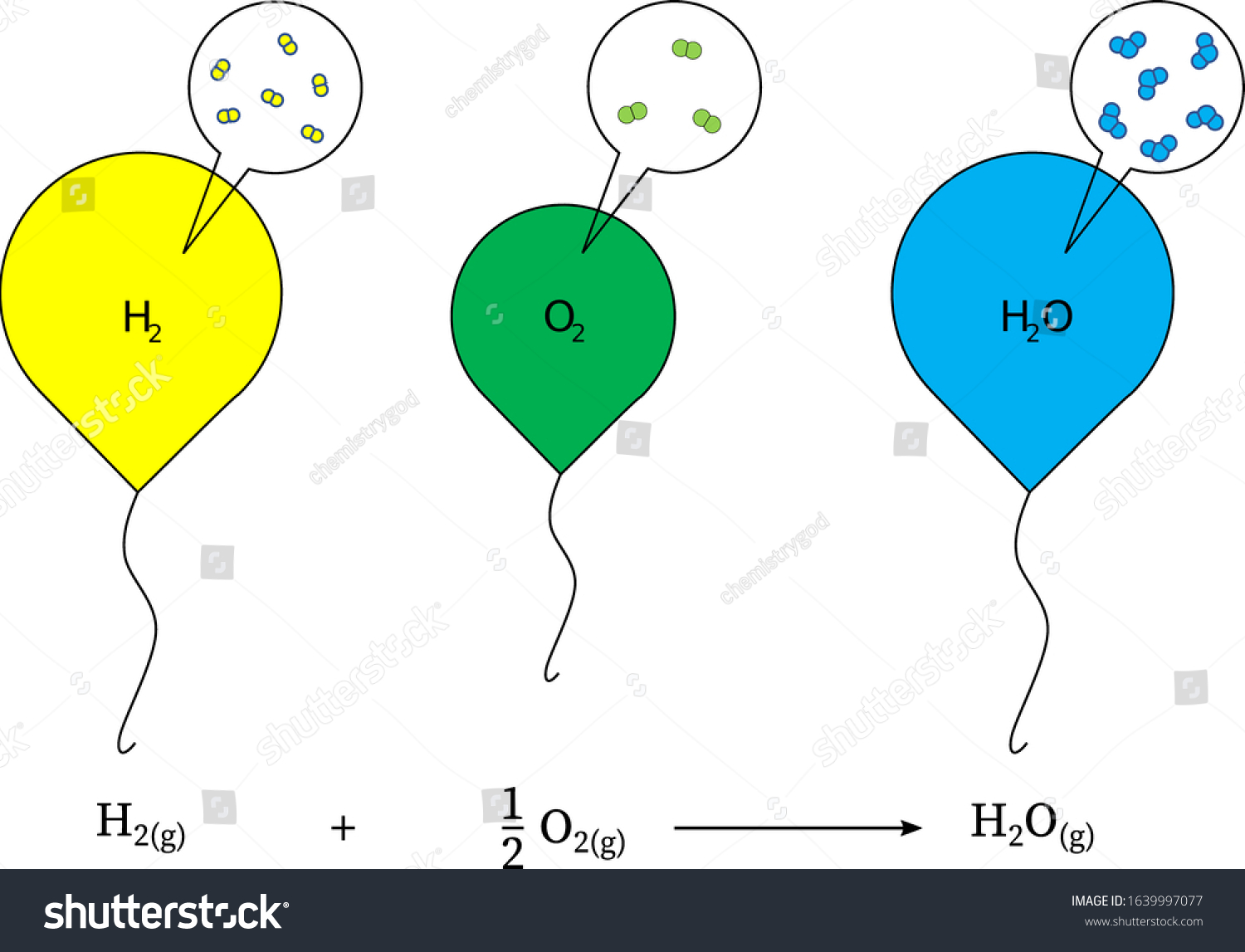
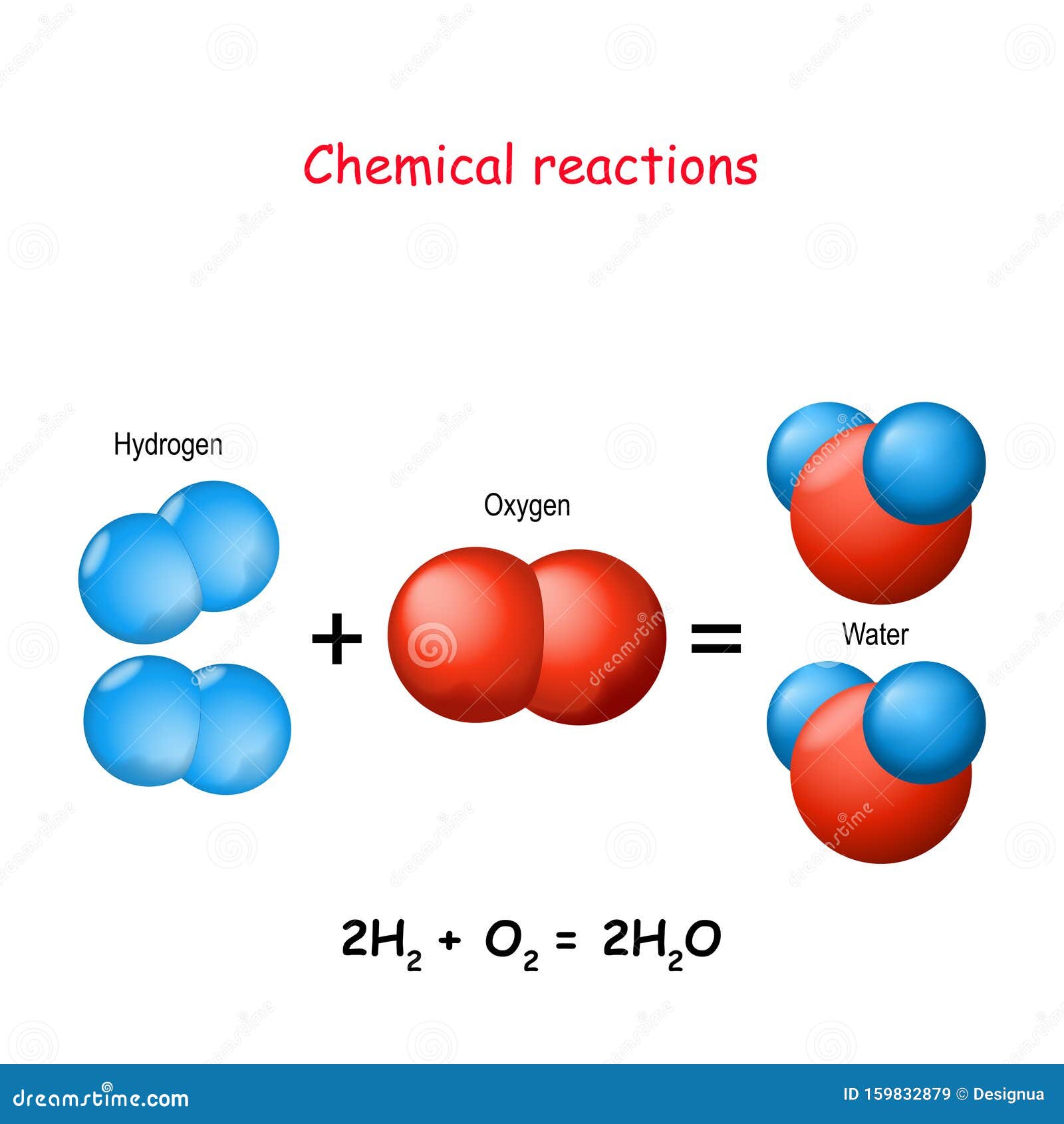
Hydrogen And Oxygen React Chemically To Form Water
Hydrogen And Oxygen React Chemically To Form Water - How much water would form if 14.8 grams of hydrogen reacted with 34.8 grams of oxygen? Web what is the complete reaction between hydrogen and oxygen to form water? O 20 g o 49.60 g 20.0 g 49.6 o 50 g. Moles of dioxygen = 38.4 ⋅ g 32.0⋅ g ⋅ mol−1 = 1.2 ⋅ mol. Web hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen and hydrogen atoms. You'll get a detailed solution from a subject matter expert that helps you learn core. 2 liters of hydrogen : When two hydrogen atoms fuse with one oxygen atom to create a molecule of water, each hydrogen atom donates its single electron to the oxygen atom, resulting in 10 electrons for the oxygen, instead of eight. Hydrogen and oxygen react chemically to form water. It can act as an acid, base, reducing agent, or oxidizing agent. To produce two molecules of water (h2o), two molecules of diatomic hydrogen (h2) must be combined with one molecule of diatomic oxygen (o2). Moles of dioxygen = 38.4 ⋅ g 32.0⋅ g ⋅ mol−1 = 1.2 ⋅ mol. The reaction releases a lot of heat as you can see in this photo of the explosion of the hindenburg: Web reactions. So we can see how hydrogen in gases reacts with oxygen Water (\(h_2o\)) and hydrogen peroxide (\(h_2o_2\)). Web hydrogenation (as shown in the figure below) is a chemical reaction that results from bonding hydrogen to organic compounds through the use of catalysts. What volume of chlorine would be produced by this reaction if 7.90 m3 of oxygen were consumed? 2. Web hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen and hydrogen atoms. 2h2 + 02 → 2h20. In this problem, which word equation shows hydrogen reacting with oxygen to form water, so student, hydrogen gas when react with oxygen gas taken form water which is in liquid form. Web. The balanced chemical equation for the reaction is: Hydrogen and oxygen react chemically to form water. Hydrogen and oxygen react chemically to form water. It can act as an acid, base, reducing agent, or oxidizing agent. Oxygen reacts with hydrogen to produce two compounds: Water (\(h_2o\)) and hydrogen peroxide (\(h_2o_2\)). Web updated on june 03, 2020. Hydrogen and oxygen react chemically to form water. Web the actual reaction to make water is a bit more complicated: You'll get a detailed solution from a subject matter expert that helps you learn core. Web what is the complete reaction between hydrogen and oxygen to form water? Web oxygen only exists as a molecule in our earth then how does the molecule oxygen react with molecule hydrogen to form water. Oxygen reacts with hydrogen to produce two compounds: Molar mass of hydrogen = 2.02 g/mole. Hydrogen and oxygen form water. In english, the equation says: Web hydrogen and oxygen gases react to form water according to the chemical equation: Web oxygen only exists as a molecule in our earth then how does the molecule oxygen react with molecule hydrogen to form water. This problem has been solved! Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can. Web we need a stoichiometric equation for water synthesis: Hydrogen and oxygen react chemically to form water. Oxygen reacts with hydrogen to produce two compounds: Web hydrogen and oxygen react chemically to form water. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. Water is the common name for dihydrogen monoxide or h 2 o. Web if the central ion is positive (a cation), then the negatively charged oxygen atoms of the water molecules are pointed toward it; Hydrogen and oxygen react chemically to form water. So we can see how hydrogen in gases reacts with oxygen Web oxygen only exists as a. 2 h 2 + o 2 → 2 h 2 o. Web when the gases dihydrogen sulfide and oxygen react, they form the gases sulfur dioxide and water vapor. Energy will be released in the process. How much water would form if 14.8 grams of hydrogen reacted with 34.8 grams of oxygen? How many grams of sulfur dioxide can be. Web hydrogenation (as shown in the figure below) is a chemical reaction that results from bonding hydrogen to organic compounds through the use of catalysts. Web when molecular hydrogen (h 2) and oxygen (o 2) are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form either water. Moles of dioxygen = 38.4 ⋅ g 32.0⋅ g ⋅ mol−1 = 1.2 ⋅ mol. Energy will be released in the process. Water (\(h_2o\)) and hydrogen peroxide (\(h_2o_2\)). The molecule is produced from numerous chemical reactions, including the synthesis reaction from its elements, hydrogen, and oxygen. How many grams of oxygen are needed to react with 2.50 g of dihydrogen sulfide? Moles of dihydrogen = 4.8 ⋅ g 2.01 ⋅ g ⋅ mol−1 = 2.39 ⋅ mol. How do we get there? 2 liters of hydrogen : Web hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen and hydrogen atoms. You'll get a detailed solution from a subject matter expert that helps you learn core. Web what is the complete reaction between hydrogen and oxygen to form water? Write down the balanced equation that represents this reaction. Web updated on june 03, 2020. 2h2 + 02 → 2h20. Web we would like to show you a description here but the site won’t allow us. How much water would form if 14.8 grams of hydrogen reacted with 34.8 grams of oxygen? When two hydrogen atoms fuse with one oxygen atom to create a molecule of water, each hydrogen atom donates its single electron to the oxygen atom, resulting in 10 electrons for the oxygen, instead of eight. 2 h 2 + o 2 → 2 h 2 o.Reaction of Hydrogen and Oxygen in New compounds. Water molecule that
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy
Explain how the model you developed shows that when oxygen combines
Hydrogen and oxygen combine in the ratio of 18 by mass to form water
The Reaction Of Hydrogen And Oxygen To Form Water
Balancing equations Hydrogen and oxygen react to form water YouTube
Chemical Reactions and Equations CK12 Foundation
PPT Ch. 1 Chemical Foundations · Chemistry An Overview PowerPoint
Water Reaction Hydrogen React Oxygen Form ภาพประกอบสต็อก 1639997077
Water Molecule. Oxygen And Hydrogen Cartoon Vector
Related Post: