Hydrochloric Acid And Sodium Hydroxide React To Form
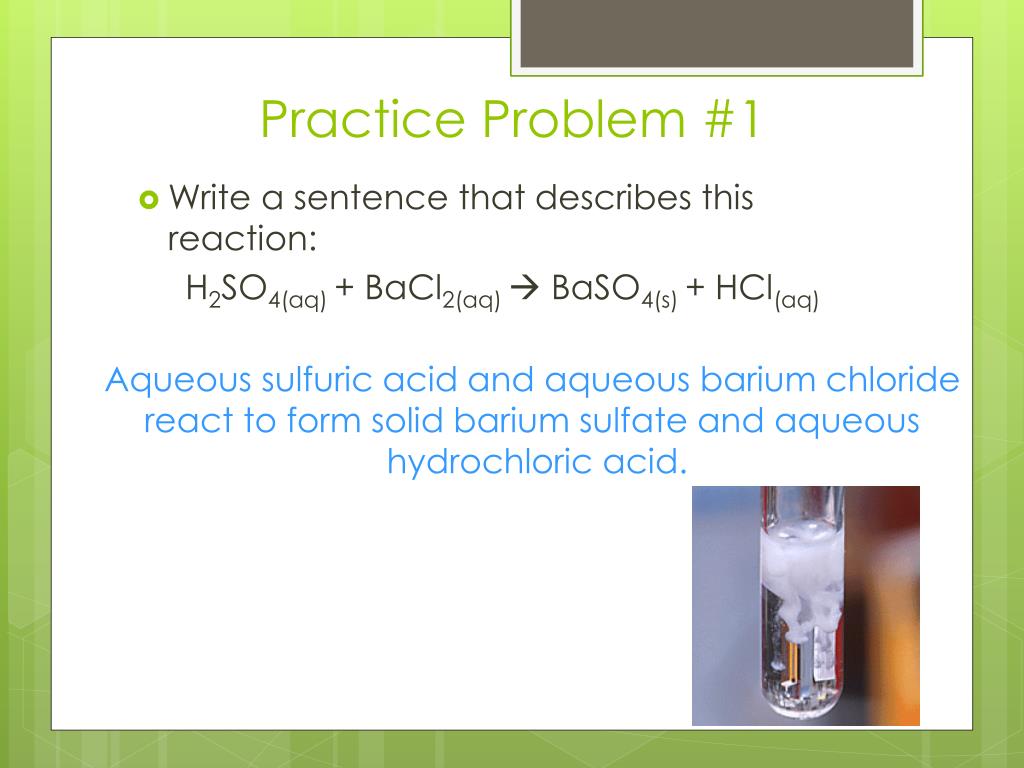
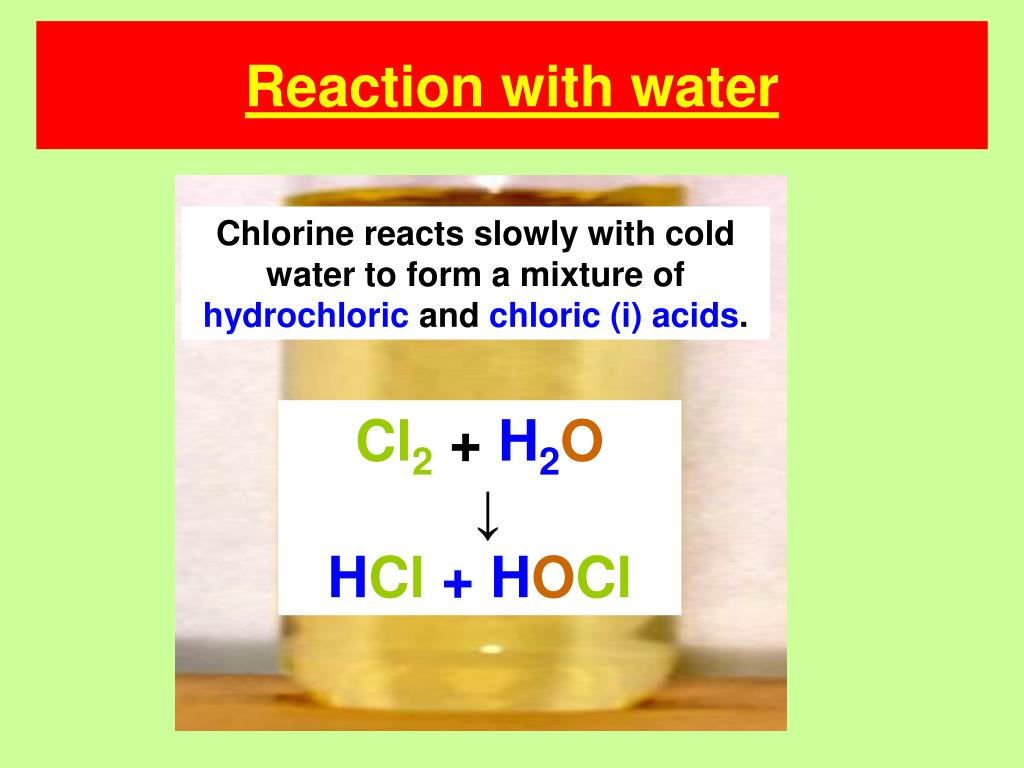
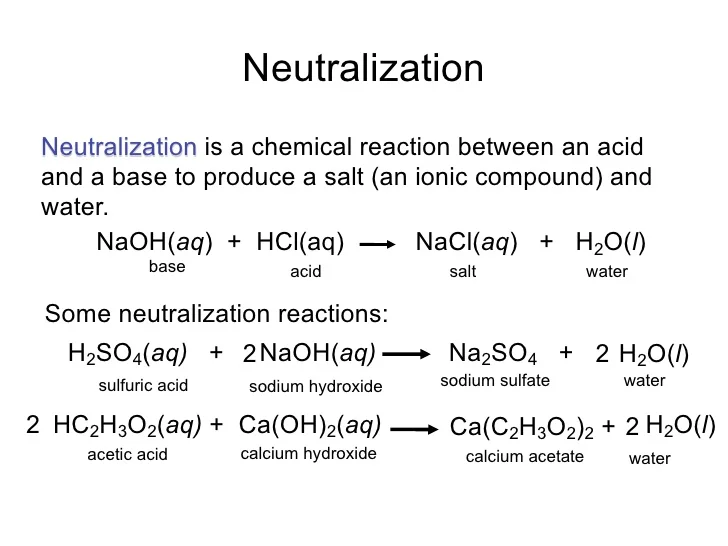
Hydrochloric Acid And Sodium Hydroxide React To Form - Bismuth(iii) chloride solution reacts with hydrochloric acid. Web hydrochloric acid and sodium hydroxide react to form salt (nacl) and water (h2o). Yes it is, it can be helpful to think of water as hydrogen hydroxide to see why this is true. Aqueiys hydrochloric acid (hcl) reacts with solid sodium hydroxide (naoh) to produce aqueous sodium chloride (nacl) and liquid water (h2o). Which statement must be true of the reaction? So, hydrochloric acid reacts with sodium hydroxide solution to form sodium chloride and water. For example, when sodium hydroxide reacts with hydrochloric acid, sodium chloride is formed: Hydrochloric acid reacts with sodium hydroxide to produce sodium chloride and water. Nacl(aq) + h 2 o(l) nah(aq) + cloh(aq) nacl(aq) + h 2 (aq) nacl(aq) + cl 2 (aq) Double diplacement reactions are summarized as follows: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.a concentrated solution of. So, hydrochloric acid reacts with sodium hydroxide solution to form sodium chloride and water. Web 1 day agothe limiting reactant is the reactant that is completely consumed in the reaction and determines the amount of product formed. The balanced chemical equation for the reaction between.. Web chemistry chemical reactions double replacement reactions. John’s school vs osei tutu shs vs opoku ware school Web hydrochloric acid and sodium hydroxide can be combined to form sodium chloride and water. Web study with quizlet and memorize flashcards containing terms like sodium hydroxide (naoh) and hydrochloric acid (hcl) react to form sodium chloride (nacl) and water according to the. Calculate the minimum mass of hydrochlaric acid that couid be left over by the chemical reaction, round your answer. Sodium hydroxide reacts with protic acids to produce water and the corresponding salts. Web hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Web acids react with most metals to form a salt and hydrogen gas.. Web study with quizlet and memorize flashcards containing terms like sodium hydroxide (naoh) and hydrochloric acid (hcl) react to form sodium chloride (nacl) and water according to the equation: If 20.6 g of sodium hydroxide reacts with an excess of hydrochloric acid, how many grams of sodium chloride are produced? Hcl + naoh → nacl + h2o. Web we would. Hydrochloric acid and sodium hydroxide react to form select one: Web hydrochloric acid (hcl) reacts with sodium hydroxide (naoh) to form sodium chloride (nacl) and water. Web this chemistry video tutorial explains how to write the net ionic equation between sodium hydroxide and hydrochloric acid. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.a concentrated solution of.. Sodium hydroxide reacts with protic acids to produce water and the corresponding salts. The total mass of hydrochloric acid and sodium hydroxide will result in a lower total mass of sodium chloride and water. Web in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Hcl + naoh → nacl +. Acetic acid, a weak acid that does not ionize completely in water. The mass of sodium hydroxide will result in the same mass of sodium chloride. Web hydrochloric acid and sodium hydroxide can be combined to form sodium chloride and water. Hcl + naoh → nacl + h2o. By the physical state of substances, it is homogenous. Sodium acetate, a salt formed from a weak acid (acetic acid) and a strong base (sodium hydroxide). Solutions of iron (iii) chloride and silver nitrate are mixed. An acid reacts with a base to form salt and water. Hydrochloric acid reacts with sodium hydroxide to produce sodium chloride and water. Web hydrochloric acid and sodium hydroxide interact, resulting in salt. Web hydrochloric acid and sodium hydroxide react to form _____. Naoh(aq) + hcl(aq) → nacl(aq) + h 2 o(l) in general, such neutralization reactions are represented by one simple net ionic equation: They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Yes it is, it can be helpful to think of water as hydrogen. Aquecus hydrochloric acid (hcl) will react with solid sodium hydroxide ( naoii ) to produce aqueous sodium chlopride (nacl) and liquid water (h2o) suppose 25. Web hydrochloric acid and sodium hydroxide react to form salt (nacl) and water (h2o). It also explains how to predict the products of this acid base. By the type of interacting substances, it is a. An acid reacts with a base to form salt and water. Web sodium hydroxide solution is treated with acetic acid to form sodium acetate and water. if 0.986g of sodium chloride is produced from the reaction of 3.3g of hydrochloric acid and 1.5 g of sodium hydroxide, calculate the percent yield of sodium chloirde. Web we would like to show you a description here but the site won’t allow us. Web study with quizlet and memorize flashcards containing terms like sodium hydroxide (naoh) and hydrochloric acid (hcl) react to form sodium chloride (nacl) and water according to the equation: Web we would like to show you a description here but the site won’t allow us. Which statement must be true of the reaction? For example, zinc metal reacts with hydrochloric acid,. Solutions of iron (iii) chloride and silver nitrate are mixed. Nacl (aq) + h2 (aq) nacl (aq) + cl2 (aq) nah (aq) + cloh (aq) nacl (aq) + h2o (1) previous question next question. Sodium hydroxide reacts with hydrochloric acid. Naoh(aq) + hcl(aq) → nacl(aq) + h 2 o(l) in general, such neutralization reactions are represented by one simple net ionic equation: Sodium chloride, a neutral salt. + 2h20l at 298 k, the standard enthalpy change, ah,, for. Hydrochloric acid is an acid while sodium hydroxide is a base. Bismuth(iii) chloride solution reacts with hydrochloric acid. This is called a neutralisation reaction. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.a concentrated solution of. Potassium carbonate solution reacts with a solution of cobalt(ii) bromide. The correct option is d sodium chloride.Acid/Base Neutralization Reaction for NaOH + HCl (Sodium hydroxide
Neutralisation Reaction of Sodium Hydroxide and Hydrochloric Acid YouTube
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Sodium Hydroxide Reacts with Hydrochloric Acid Stock Image C036
33++ Hydrochloric Acid And Sodium Hydroxide Reaction Insende
Diagram shows the apparatus setup to study the reaction between sodium
PPT Reactions of chlorine with water and sodium hydroxide. PowerPoint
🎉 Hydrochloric acid and sodium hydroxide. What is the equation for
[Solved] Hydrochloric acid and sodium hydroxide react to produce water
33++ Hydrochloric Acid And Sodium Hydroxide Reaction Insende
Related Post: