Hybrid Orbitals Overlap To Form
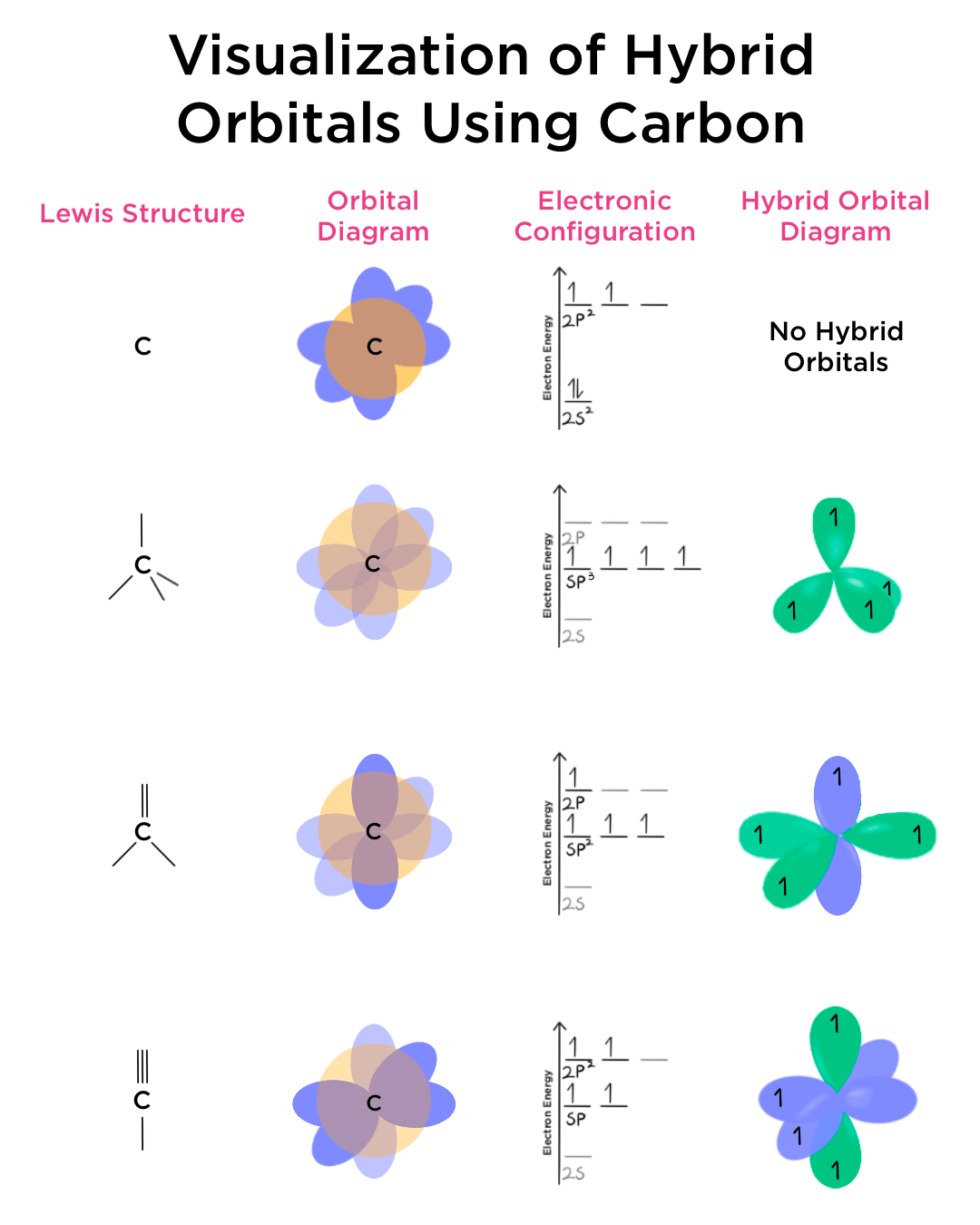
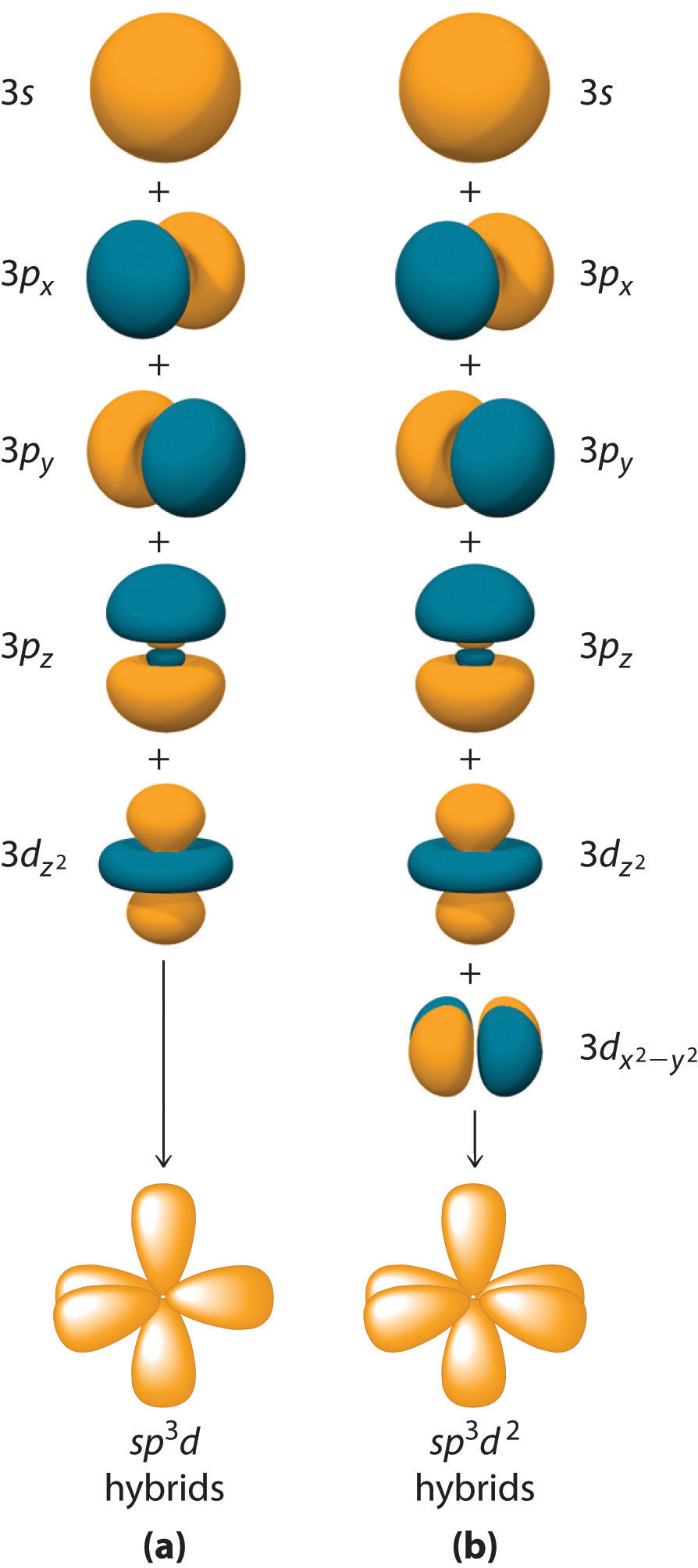
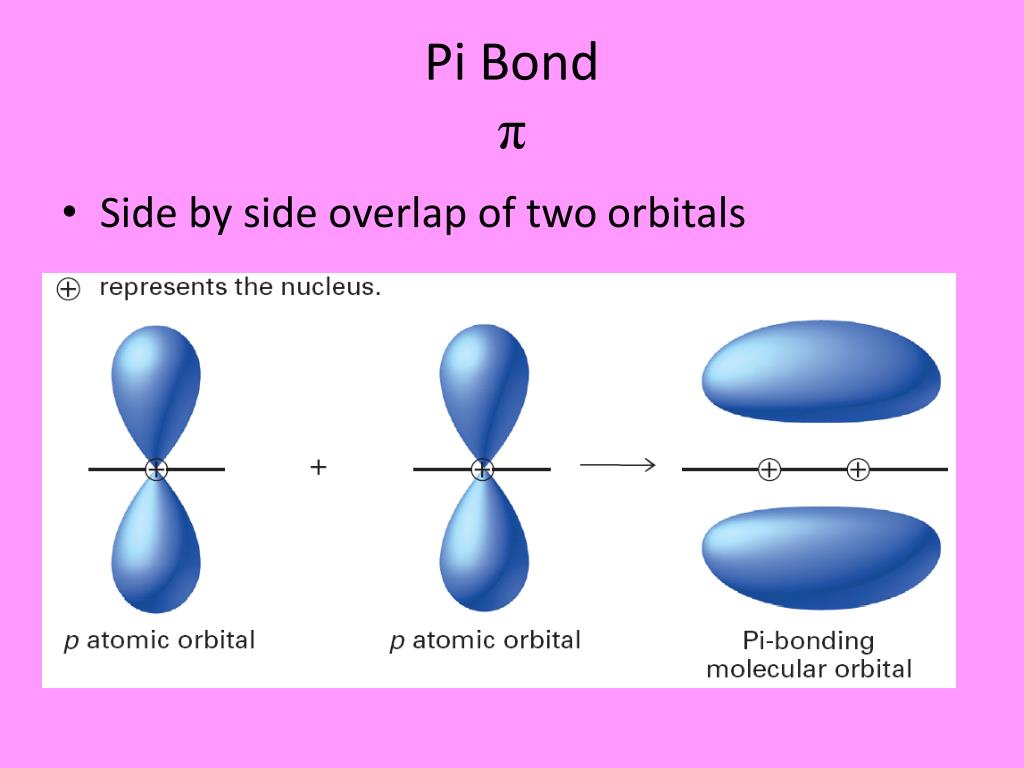
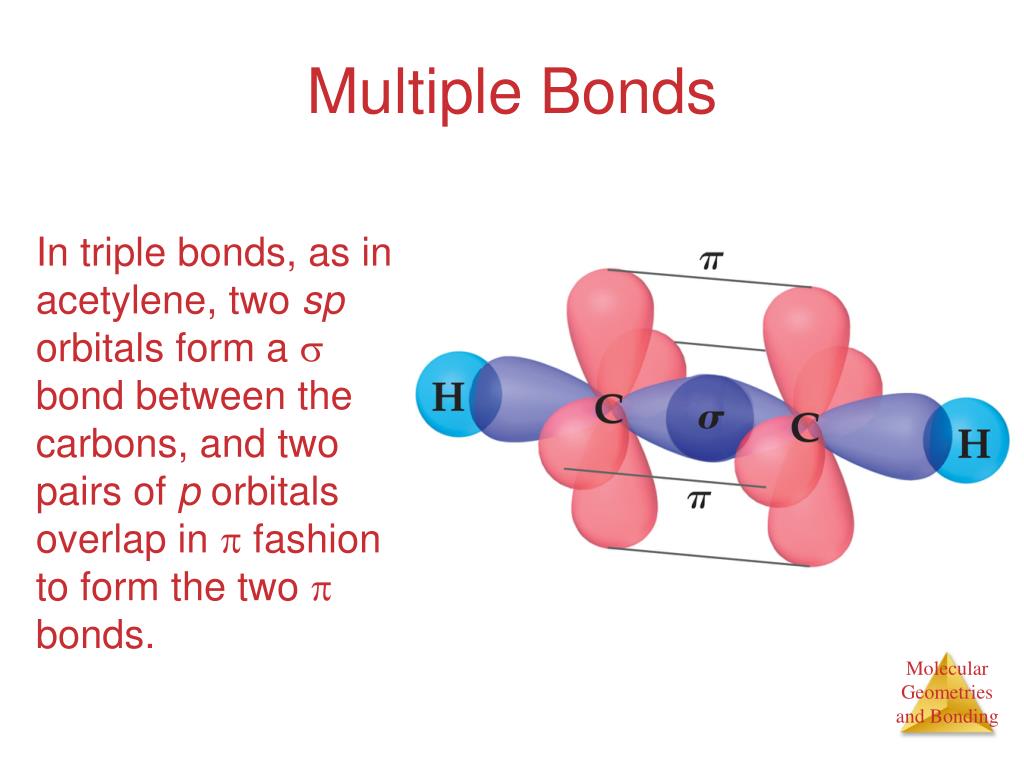
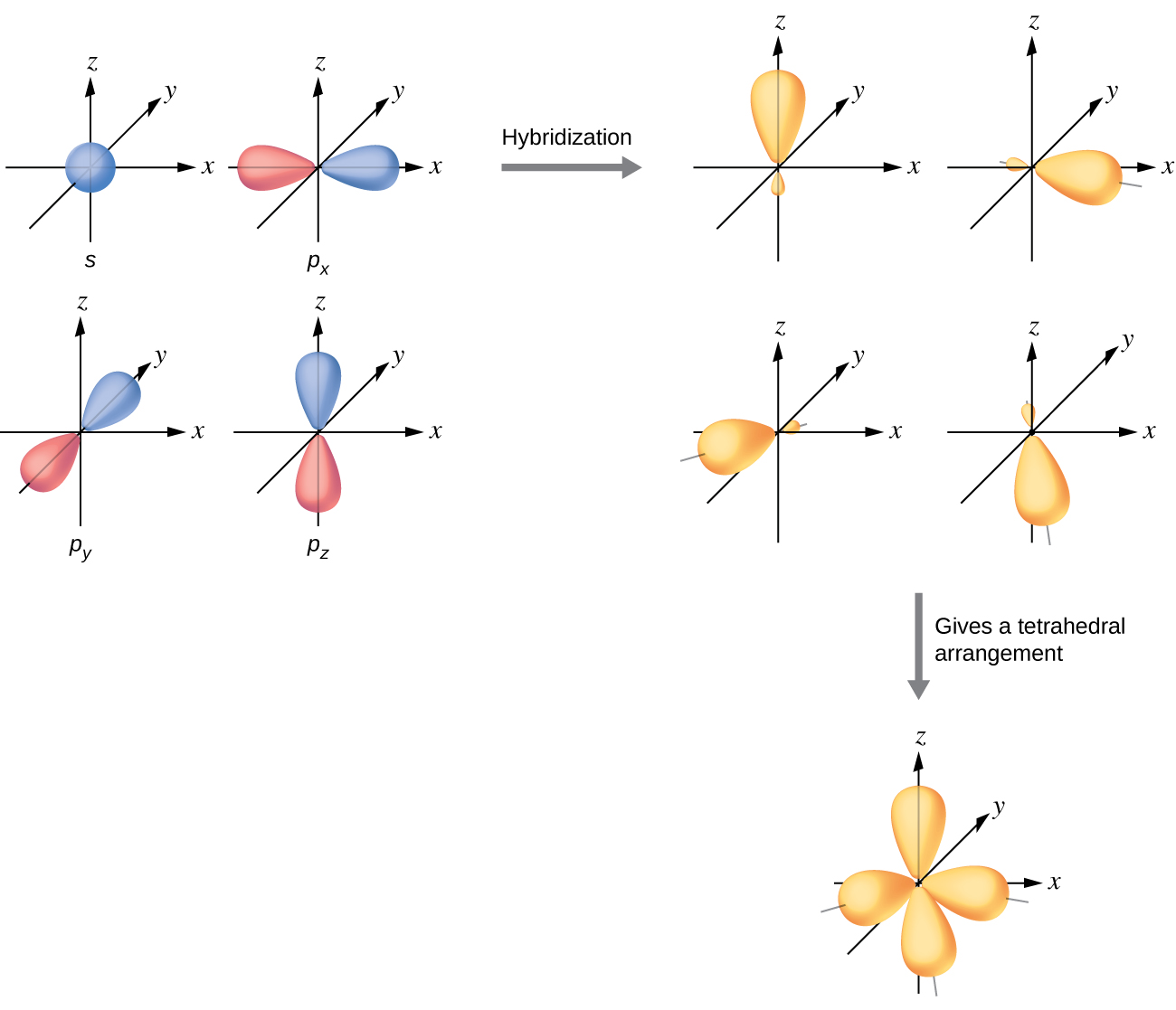
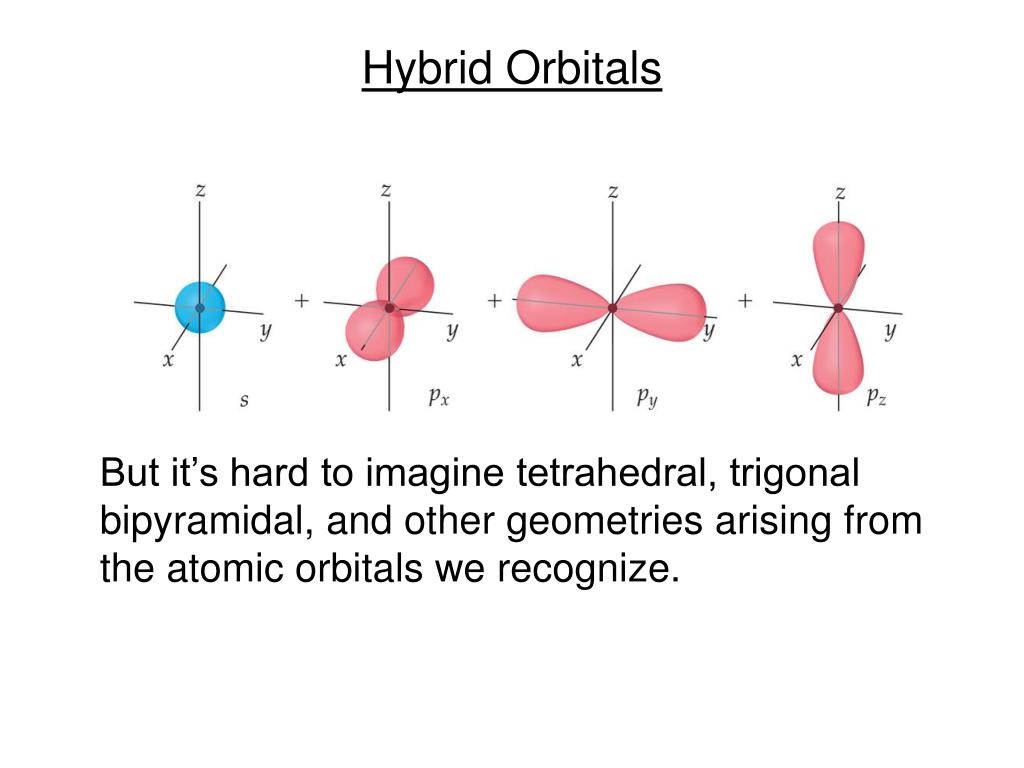
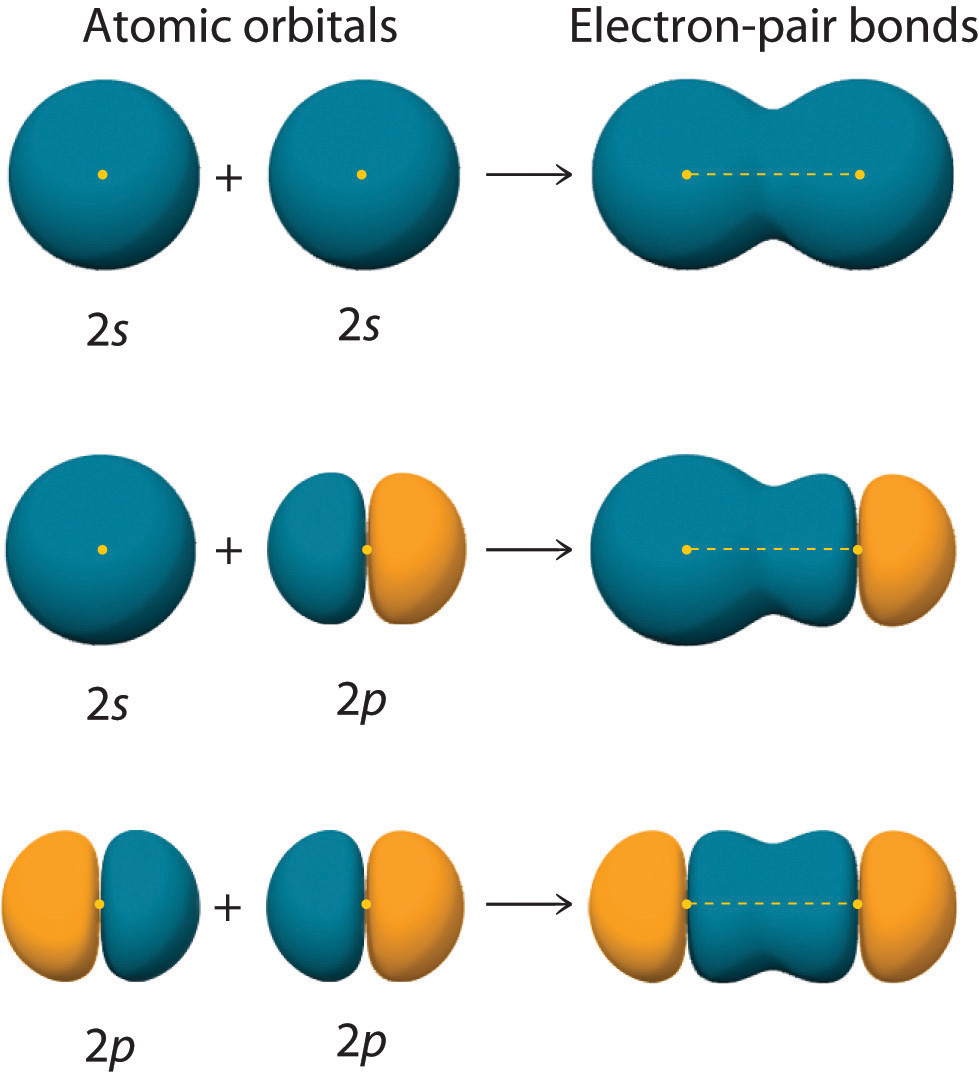
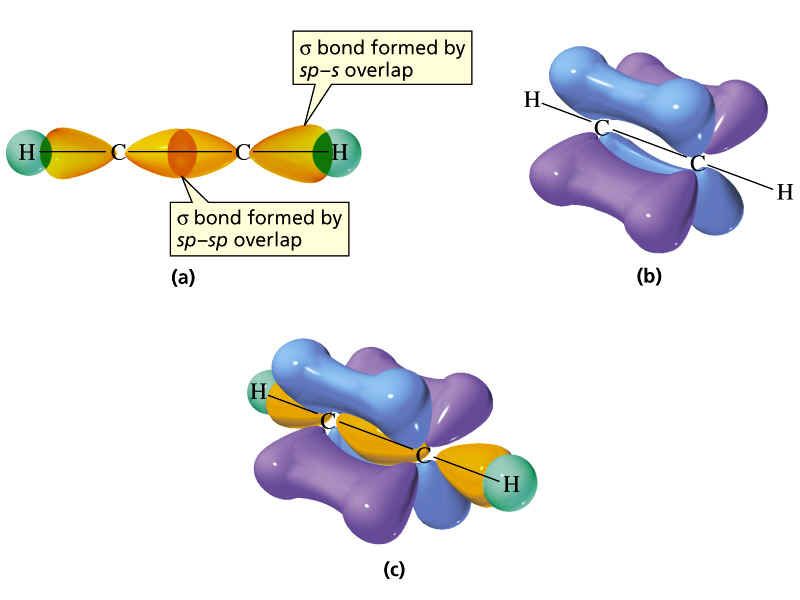
Hybrid Orbitals Overlap To Form - Unhybridized orbitals overlap to form π bonds. Web after hybridization, however, each carbon still has one unhybridized 2 pz orbital that is perpendicular to the hybridized lobes and contains a single electron (part. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. A solution to this problem was proposed by linus pauling, who. Web as they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. In the following sections, we shall discuss the common types of hybrid. This lowers the potential energy of the system, as new, attractive. Web hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds. Unhybridized orbitals overlap to form π bonds. In the following sections, we shall discuss the common types of hybrid. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. Web hybrid orbitals overlap to form σ bonds. It is difficult to explain the shapes of even the simplest molecules with atomic orbitals. Web science chemistry chemistry questions and answers hybrid orbitals overlap to form__bonds. Web as they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to form a π bond. Unhybridized orbitals overlap to form π. Web chemistry questions and answers. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. Web how the atomic orbitals are combined to give sp 3 orbitals is a bit complicated, so we will consider the simpler case of the sp hybrid formed from. Web sigma bonds are the first bonds that form (in the. You'll get a detailed solution from a subject matter expert. Web sigma bonds are the first bonds that form (in the realm of covalent bonding) and you can imagine them as a direct connection between the two nuclei involved. In the following sections, we shall discuss the common types of hybrid. This type of hybridization is. A solution to this. Web as they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. Web how the atomic orbitals are combined to give sp 3 orbitals is a bit complicated, so we will consider the simpler case of the sp hybrid formed from. Unhybridized orbitals overlap to form bonds. Unhybridized orbitals overlap to form π. Figure 8.23 in the ethene molecule, c 2 h 4, there are (a) five. This lowers the potential energy of the system, as new, attractive. Web science chemistry chemistry questions and answers hybrid orbitals overlap to form__bonds. Web hybrid orbitals overlap to form σ bonds. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each. Web sigma bonds are the first bonds that form (in the realm of covalent bonding) and you can imagine them as a direct connection between the two nuclei involved. Unhybridized orbitals overlap to form π bonds. It is difficult to explain the shapes of even the simplest molecules with atomic orbitals. Web in sp³ hybridization, one s orbital and three. Web hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form bonds. Web in sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is. Unhybridized orbitals overlap to form π bonds. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to form a π bond. Unhybridized orbitals overlap to form π bonds. Web science chemistry chemistry questions and answers hybrid orbitals overlap to form__bonds. Are a type of atomic orbital that results when two or more atomic orbitals of an isolated atom. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to form a π bond. Web we take the two higher energy p orbital electrons and the two lower energy s orbital electrons and meld them into four equal energy sp 3 ( 1s + 3 p orbitals = sp 3) hybrid.. This problem has been solved! Web hybrid orbitals overlap to form σ bonds. Web sigma bonds are the first bonds that form (in the realm of covalent bonding) and you can imagine them as a direct connection between the two nuclei involved. Web after hybridization, however, each carbon still has one unhybridized 2 pz orbital that is perpendicular to the hybridized lobes and contains a single electron (part. Web in sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. Web hybrid orbitals overlap to form σ bonds. Web science chemistry chemistry questions and answers hybrid orbitals overlap to form__bonds. You'll get a detailed solution from a subject matter expert. Unhybridized orbitals overlap to form π bonds. In the following sections, we shall discuss the common types of hybrid. This lowers the potential energy of the system, as new, attractive. Web all orbitals in a set of hybrid orbitals are equivalent in shape and energy. Are a type of atomic orbital that results when two or more atomic orbitals of an isolated atom mix (the number of hybrid orbitals on a covalently bonded atom is. Web chemistry questions and answers. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to form a π bond. Unhybridized orbitals overlap to form bonds. It is difficult to explain the shapes of even the simplest molecules with atomic orbitals. This type of hybridization is. Which hybrid orbitals overlap to form the sigma bonds between the indicated atoms in xanthine?which of the following molecules is. Figure 8.23 in the ethene molecule, c 2 h 4, there are (a) five.hybridization Hybrid orbitals forming molecular orbitals Chemistry
Hybrid Orbitals — Overview & Examples Expii
9.5 Hybrid Orbitals Chemistry LibreTexts
Hybridization of Orbitals Chemistry Topics Chemistry classroom
PPT Chapter 8 Lecture 8.5 Hybridization PowerPoint Presentation, free
PPT Hybrid Orbitals PowerPoint Presentation, free download ID6008276
8.2 Hybrid Atomic Orbitals Chemistry LibreTexts
PPT Overlap and Bonding PowerPoint Presentation, free download ID
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts
Orbital Hybridization Chemistry Skills
Related Post: