How Many Single Covalent Bonds Can Halogens Form
How Many Single Covalent Bonds Can Halogens Form - What is the correct name for. Web how many covalent bonds are formed? The bonds in these diatomic molecules are non. Oxygen and other atoms in group 6a obtain an octet by forming two covalent bonds. Web the halogens also form single covalent bonds in their diatomic molecules. Terms in this set (51) which type of molecular shape is shown by this model? An atom of any halogen, such as fluorine, has seven valence electrons. Typically, the atoms of group 4a form 4. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence. Web how many single covalent bonds can halogens form? Web how many single covalent bonds can halogens form? Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). 1 sigma bond only c. Which is true of the bond length of these covalent molecules? What is the correct name for. Which is true of the bond length of these covalent molecules? Typically, the atoms of group 4a form 4. Web in compounds wherein the halogen atom is involved in the formation of one covalent bond, by far the most common case, there is a region of higher electron. Halogens form diatomic molecules (of the form x2, where x denotes a. 1 sigma bond & 1 or 2 pi bonds. Typically, the atoms of group 4a form 4. Web how many single covalent bonds can halogens form? Web how many single covalent bonds can halogens form? 1 sigma bond only c. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). An atom of any halogen, such as fluorine, has seven valence electrons. 1 sigma bond & 1 pi bond d. Oxygen and other atoms in group 6a obtain an octet by forming two covalent bonds. There are different kinds of covalent bonds: The bonds in these diatomic molecules are non. Web how many covalent bonds are formed? Web the halogens also form single covalent bonds in their diatomic molecules. 1 sigma bond and 1 pi bond. Typically, the atoms of group 4a form 4. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The potential energy of two separate hydrogen atoms (right) decreases as. B) n (three lines) n. A single covalent bond is when two atoms share a. Web in compounds wherein the halogen atom is involved in the formation of one covalent bond, by far. Terms in this set (51) which type of molecular shape is shown by this model? Web the halogens also form single covalent bonds to produce diatomic molecules. 1 sigma bond & 1 or 2 pi bonds. A single covalent bond is when two atoms share a. The carbon atom is unique among elements in its tendency to form extensive networks. What is the correct name for. Double covalent bonds consist of which of these? The bonds in these diatomic molecules are non. 1 sigma bond & 2 pi bonds. Web the halogens also form single covalent bonds to produce diatomic molecules. How many single covalent bonds can halogens form? Web fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet by forming one covalent bond. There are different kinds of covalent bonds: Oxygen and other atoms in group 6a obtain an octet by forming two covalent bonds. Web how many single covalent bonds. 1 sigma bond & 1 pi bond d. Web how many covalent bonds are formed? Web the halogens also form single covalent bonds in their diatomic molecules. An atom of any halogen, such as fluorine, has seven valence electrons. Web the halogens also form single covalent bonds to produce diatomic molecules. Which is true of the bond length of these covalent molecules? Web the halogens also form single covalent bonds in their diatomic molecules. Typically, the atoms of group 4a form 4. 1 sigma bond and 1 pi bond. 1 sigma bond & 2 pi bonds. Oxygen and other atoms in group 6a obtain an octet by forming two covalent bonds. Typically, the atoms of group 4a form 4. The bonds in a are longer than the. The potential energy of two separate hydrogen atoms (right) decreases as. 1 sigma bond & 1 pi bond d. Which is true of the bond length of these covalent molecules? 1 sigma bond only c. What is the correct name for. Halogens form diatomic molecules (of the form x2, where x denotes a halogen atom) in their elemental states. The carbon atom is unique among elements in its tendency to form extensive networks of covalent. Terms in this set (51) which type of molecular shape is shown by this model? Web fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet by forming one covalent bond. B) n (three lines) n. An atom of any halogen, such as fluorine, has seven valence electrons. 1 sigma bond & 1 or 2 pi bonds.Single Covalent Bonds Chemistry for NonMajors
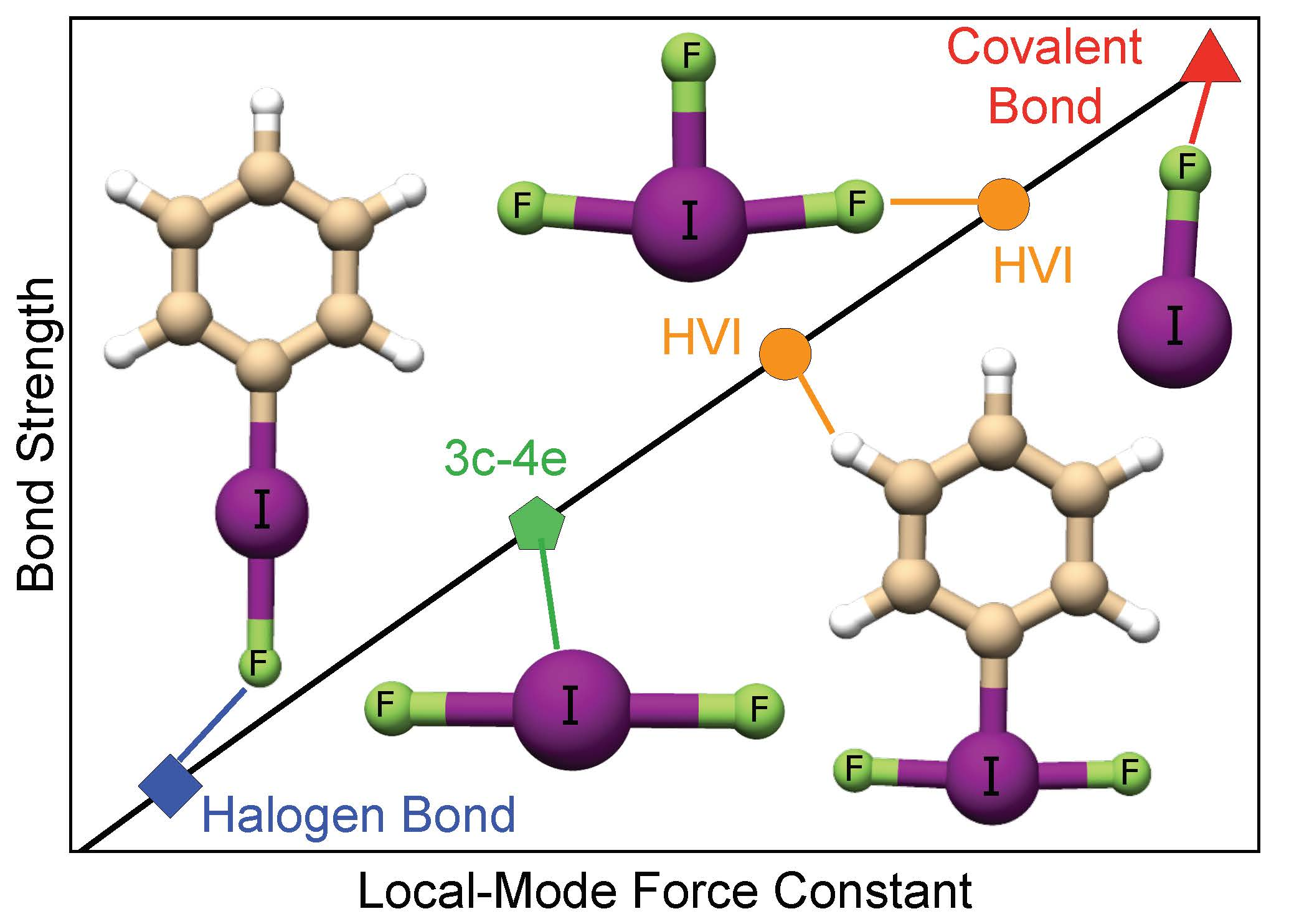
Free FullText A Continuum from Halogen Bonds to
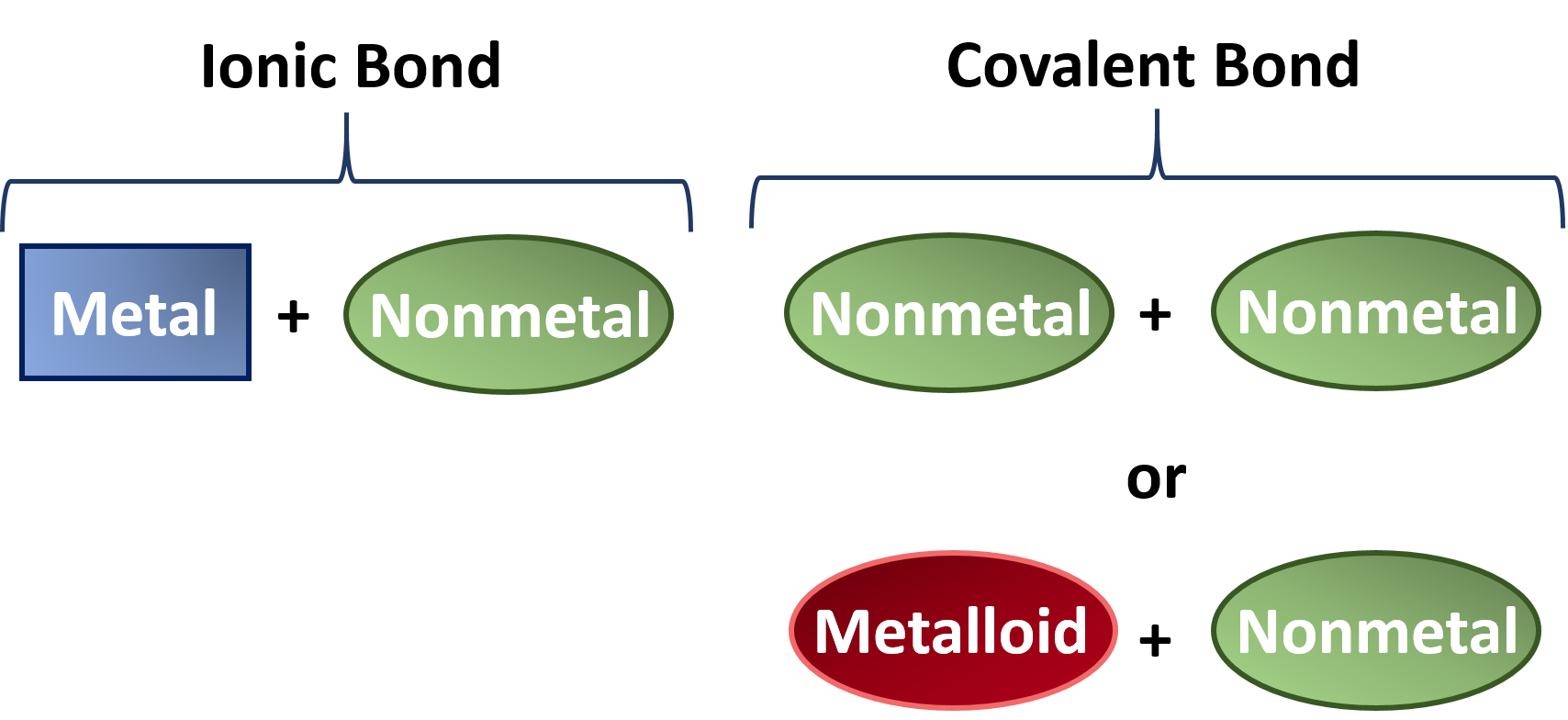
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
covalent bond Definition, Properties, Examples, & Facts Britannica
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Chemical Bonding Types of Chemical Bonds, Bond Characteristics, Enthalpy
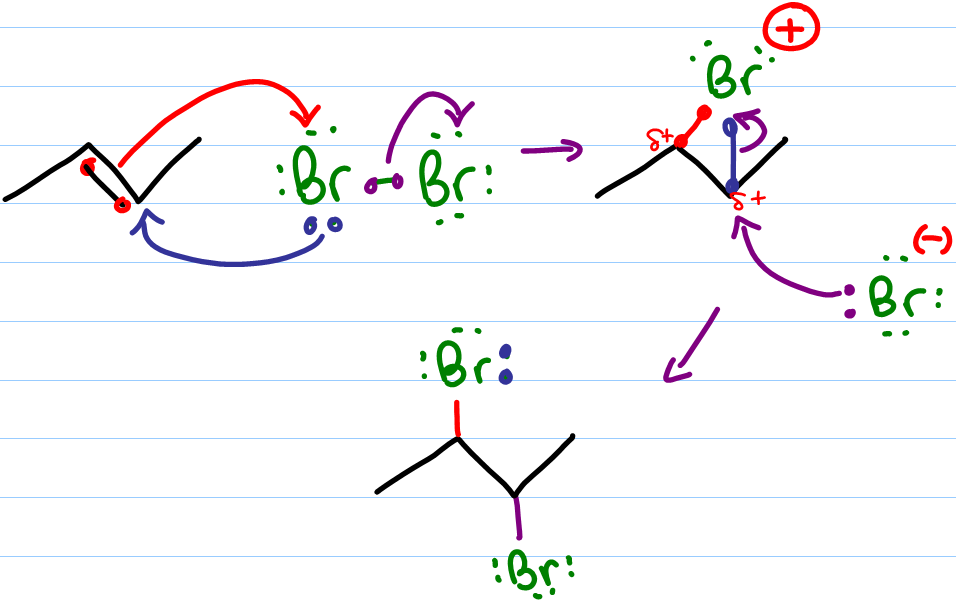
Halogenation of Alkenes Organic Chemistry Reaction Mechanism MCAT
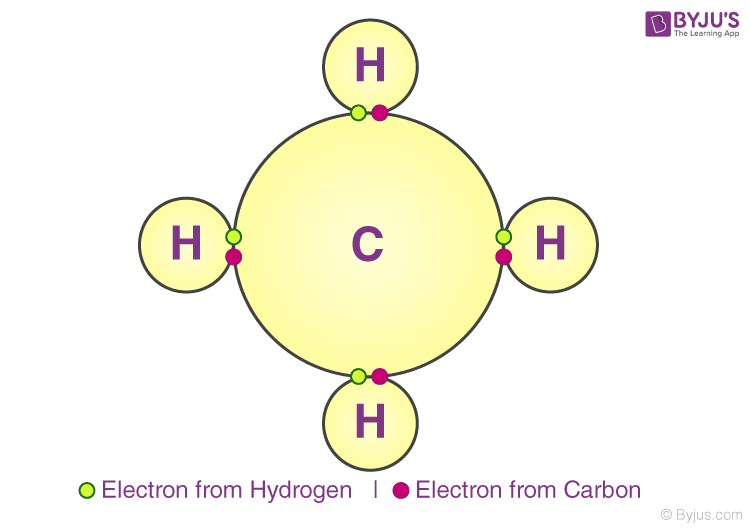
Reading Covalent Bonds Biology I
halogen Facts, Definition, Properties, & Uses Britannica
Single Covalent Bond Definition & Examples Video & Lesson Transcript
Related Post: