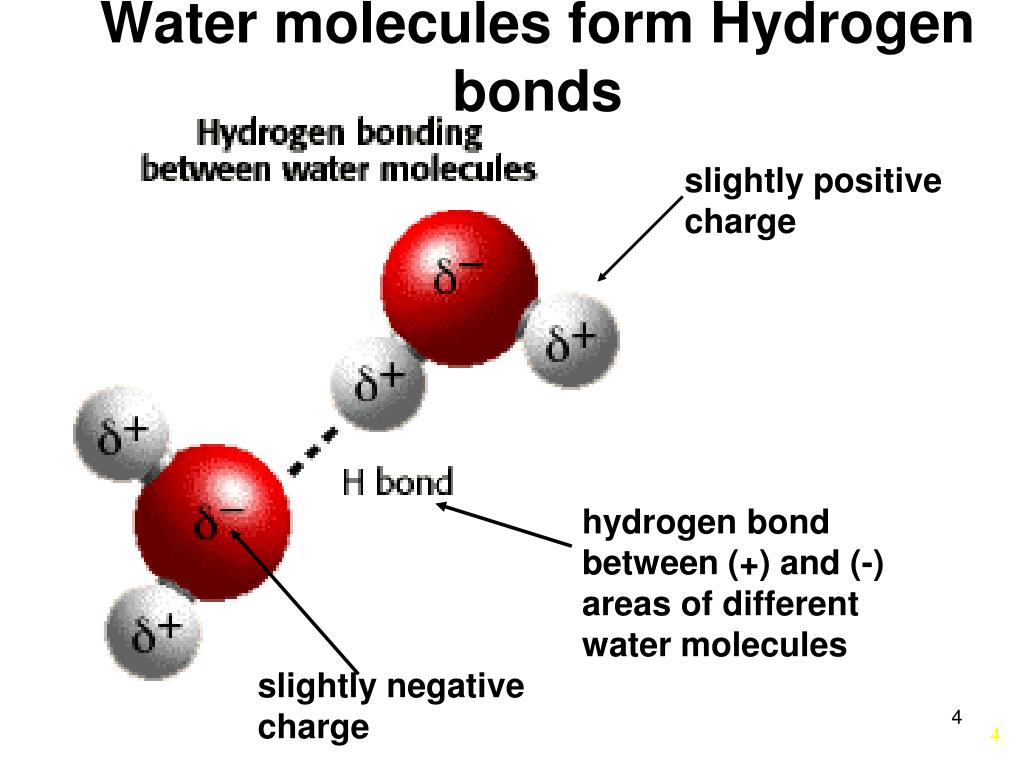
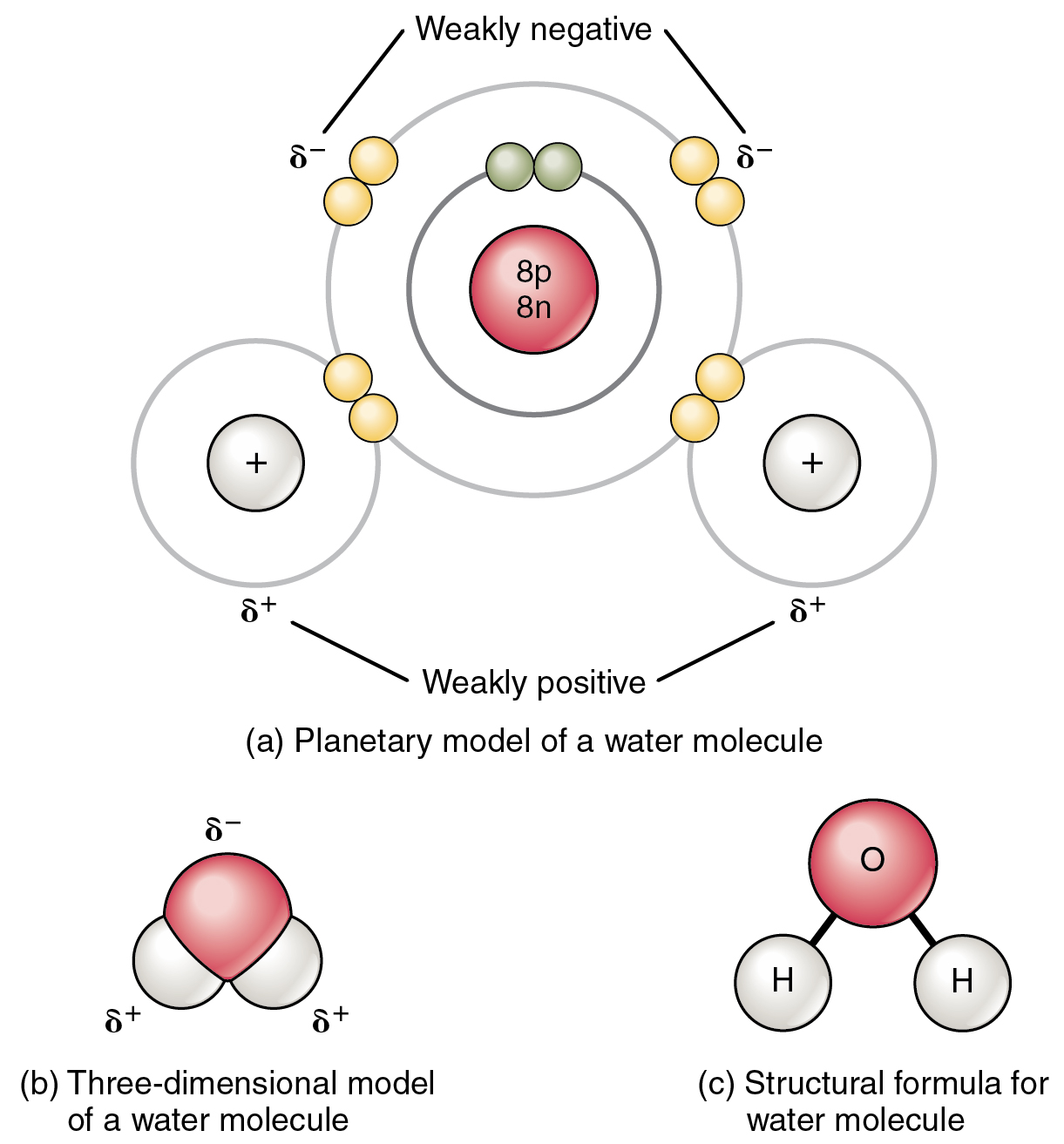
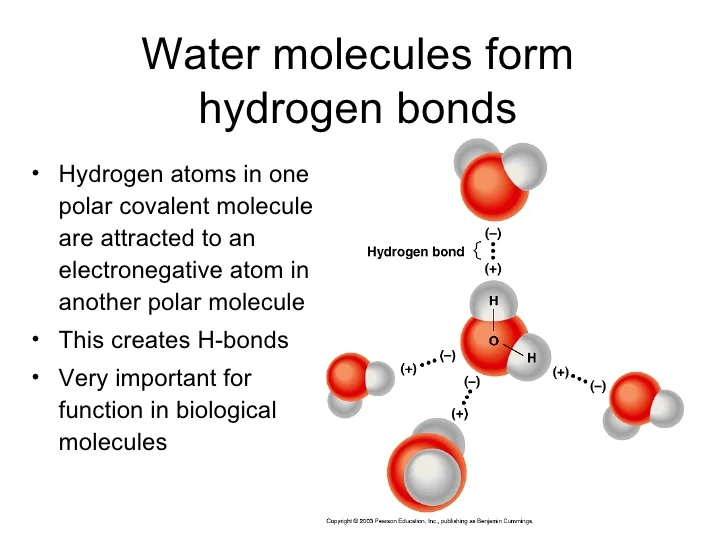
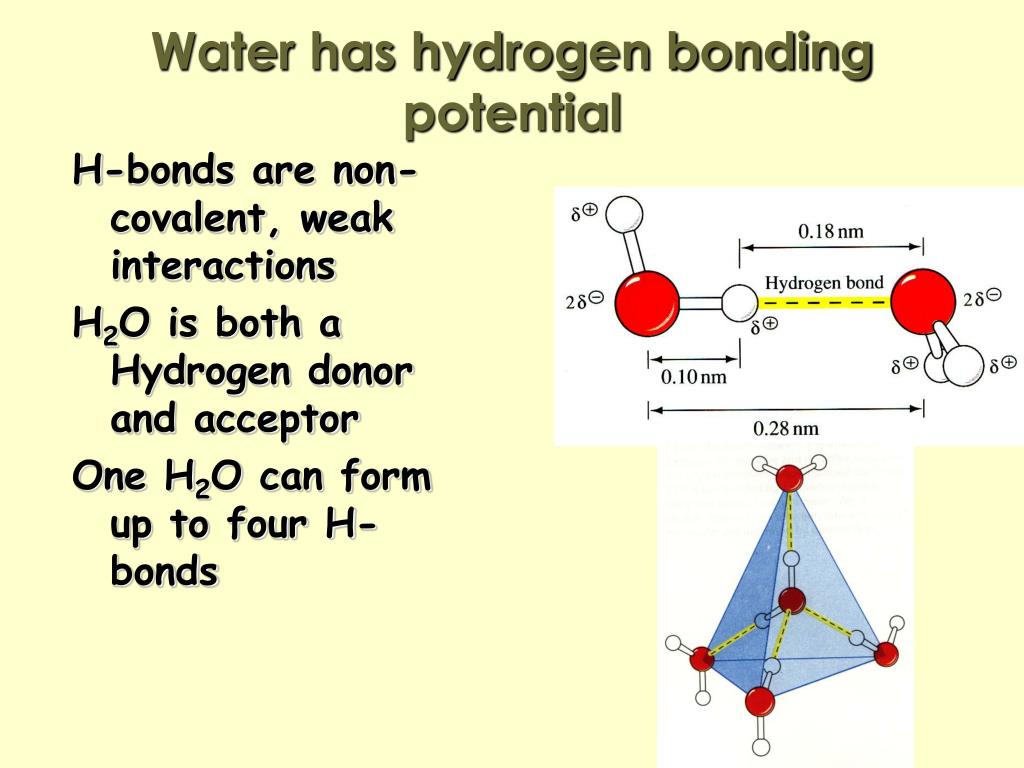
How Many Hydrogen Bonds Can Water Form
How Many Hydrogen Bonds Can Water Form - Oxygen is highly electronegative, which creates a partial negative charge on one end of the molecule, and a partial positive charge on the other. Web how many hydrogen bonds can be formed by water in solid and liquid form? Enjoy great deals and discounts on an array of products from various brands. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat capacity. Assume all possible sites are available. However, because they are exposed to air on one side, they will have fewer neighboring water molecules to. Web explain hydrogen bonding in terms of water. How many hydrogen bonds can a single water molecule form? Two given through the h atoms (towards two other h2o molecules), and two received on the o atom (from h. In h 2 o, only two of the six. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. The structure of water molecules and how they can interact to form hydrogen bonds. Web because of the strong hydrogen bonds, water molecules are able to stay condensed in the liquid state. Web how. Web the total number of hydrogen bonds formed between water molecules is 4. How many hydrogen bonds can a single water molecule form? Web how many hydrogen bonds can be formed by water in solid and liquid form? However, because they are exposed to air on one side, they will have fewer neighboring water molecules to. Oxygen is highly electronegative,. How many hydrogen bonds can a single water molecule form? In h 2 o, only two of the six. Web water as a perfect example of hydrogen bonding. Web we would like to show you a description here but the site won’t allow us. Web explain hydrogen bonding in terms of water. Each water molecule can form 2 hydrogen bonds between oxygen and the two. Positive hydrogen of one molecule attracted to negative oxygen. Web the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat capacity. Web we would like to show you a description here. Two given through the h atoms (towards two other h2o molecules), and two received on the o atom (from h. Web water as a perfect example of hydrogen bonding. So yes, we can have hydrogen. Both an oxygen atom and 2 hydrogen atoms in one molecule. The figure below shows how the bent shape, and two. Web the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat capacity. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape.. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. In h 2 o, only two of the six. Web the hydrogen bonds in. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web water as a perfect example of hydrogen bonding. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. How many hydrogen bonds can a single water molecule form?. The structure of water molecules and how they can interact to form hydrogen bonds. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Web we would like to show you a description here but the site won’t allow us. Web water is made up of. Each water molecule can form 2 hydrogen bonds between oxygen and the two. Ho 7 08 3 05 nh₂ oh Web water molecule can have/form a maximum of four hydrogen bonds: Enjoy great deals and discounts on an array of products from various brands. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does. That means that every hydrogen. Web the hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat capacity. Web the total number of hydrogen bonds formed between water molecules is 4. How many hydrogen bonds can a single water molecule form? Web because of the strong hydrogen bonds, water molecules are able to stay condensed in the liquid state. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web water molecule can have/form a maximum of four hydrogen bonds: Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web we would like to show you a description here but the site won’t allow us. Web how many hydrogen bonds can be formed by water in solid and liquid form? The figure below shows how the bent shape, and two. Both an oxygen atom and 2 hydrogen atoms in one molecule. Each water molecule can form 2 hydrogen bonds between oxygen and the two. Positive hydrogen of one molecule attracted to negative oxygen. Two given through the h atoms (towards two other h2o molecules), and two received on the o atom (from h. Web water as a perfect example of hydrogen bonding. Assume all possible sites are available.Chemical Bonds · Anatomy and Physiology
Water
Water has both a hydrogen bond and a polar covalent bond. Hydrogen
Water Review
Properties of Water Presentation Biology
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
PPT Properties of water PowerPoint Presentation, free download ID
The Unique Properties Of Water How Hydrogen Bonding Affects Our Body
Properties of Water Presentation Biology
PPT Water Chemistry & Properties of Water PowerPoint Presentation
Related Post:


.PNG)


.PNG)