How Many Hydrogen Bonds Can A Water Molecule Form
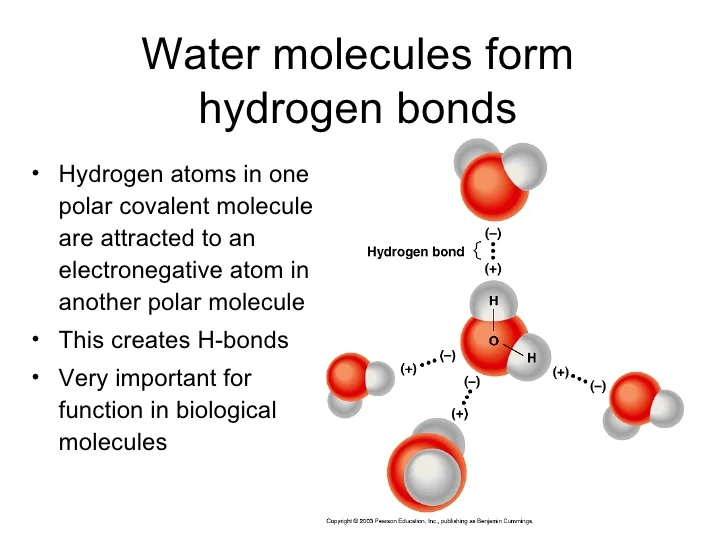
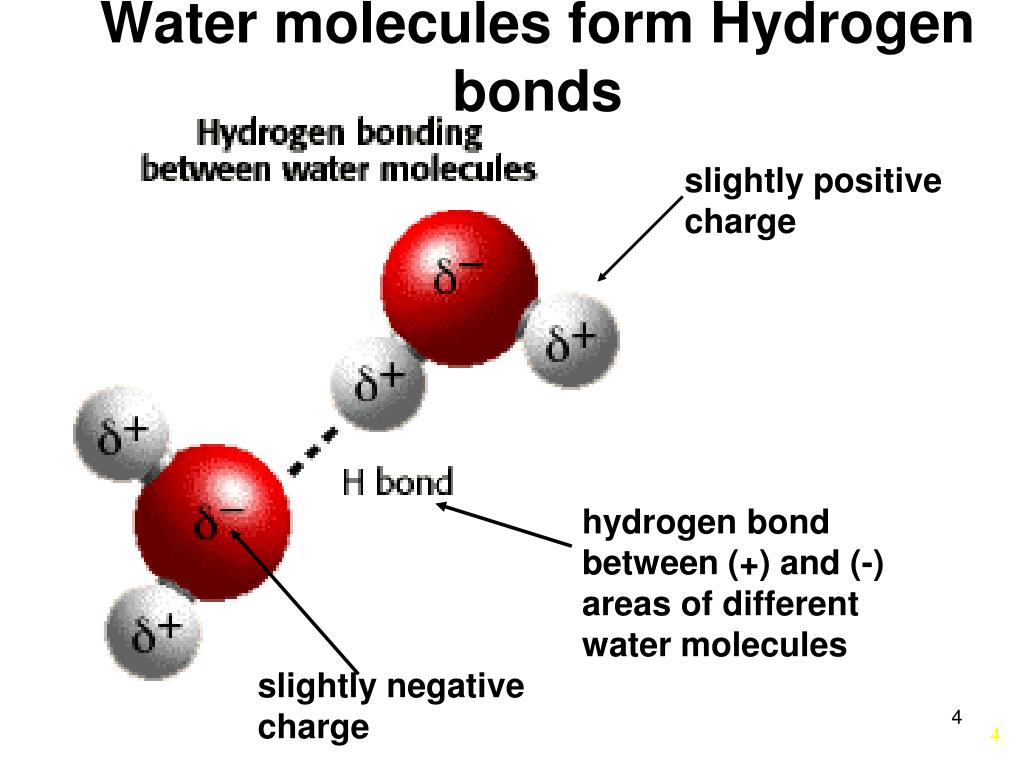
How Many Hydrogen Bonds Can A Water Molecule Form - In this question, we need to determine the maximum number of hydrogen bonds that can be formed by one molecule of water. Ad browse & discover thousands of science book titles, for less. Web because each oxygen atom has two lone pairs, it can make hydrogen bonds to the hydrogen atoms of two separate other molecules. Get solutions get solutions get. Web 8 years ago hello esther! This means each water molecule can participate in up to 4. Each water molecule is surrounded by four neighboring h 2 os. Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Polarity is the result of electronegativity. Electronegativity is the amount of pull that the members of a covalent bond exert on their shared electrons. This means each water molecule can participate in up to 4. Web water is an ideal example of hydrogen bonding. Covalent bonds are bonds that are formed when two atoms share their. The structure of water molecules and how they can interact to form hydrogen bonds. Ad browse & discover thousands of science book titles, for less. Hydrogen bonds plants transport water to their leaves through the xylem when water evaporates from the. Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Web 8 years ago hello esther! Covalent bonds are bonds that are formed when two atoms share their. The figure below shows the. Polarity is the result of electronegativity. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web 8 years ago hello esther! That means that every hydrogen. Web water also has two lone pairs and two h atoms attached to the highly electronegative oxygen. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules:. In this question, we need to determine the maximum number of hydrogen bonds that can be formed by one molecule of water. Two given through the h atoms (towards two other h2o molecules), and two received on the o atom (from. Positive hydrogen of one. Both an oxygen atom and 2 hydrogen atoms in one molecule. In this question, we need to determine the maximum number of hydrogen bonds that can be formed by one molecule of water. Web 8 years ago hello esther! Web water molecule can have/form a maximum of four hydrogen bonds: Web solutions for chapter 14 problem 118ep: Get solutions get solutions get. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. Web because each oxygen atom has two lone pairs, it can make hydrogen bonds to the hydrogen atoms of two separate other molecules. Web the 4 possible hydrogen bonds formed with a water molecule in ice. Web water molecule. Covalent bonds are bonds that are formed when two atoms share their. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as. Web solutions for chapter 14 problem 118ep: Web how many hydrogen bonds can a single water molecule form? Electronegativity is the amount of pull that the members of a covalent bond exert. Web water is an ideal example of hydrogen bonding. Benzophenone reacts with sodium borohydride in the. Web solution for how many hydrogen bonds are possible for this molecule to form? Web we would like to show you a description here but the site won’t allow us. Electronegativity is the amount of pull that the members of a covalent bond exert. Electronegativity is the amount of pull that the members of a covalent bond exert on their shared electrons. How many hydrogen bonds can form between a single ether molecule and water molecules?. Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. That means that every hydrogen. Web water also has two lone pairs and two h atoms. How many hydrogen bonds can form between a single ether molecule and water molecules?. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules:. Each water molecule is surrounded by four neighboring h 2 os. Assume all possible sites are available. Web solutions for chapter 14 problem 118ep: Every water molecule can be. Web water also has two lone pairs and two h atoms attached to the highly electronegative oxygen. Each water molecule is surrounded by four neighboring h 2 os. Web hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web solution for how many hydrogen bonds are possible for this molecule to form? Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. That means that every hydrogen. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as. This means each water molecule can participate in up to 4. The structure of water molecules and how they can interact to form hydrogen bonds. So yes, we can have hydrogen bonding between one h2o. Web because each oxygen atom has two lone pairs, it can make hydrogen bonds to the hydrogen atoms of two separate other molecules. Two given through the h atoms (towards two other h2o molecules), and two received on the o atom (from. Web water molecule can have/form a maximum of four hydrogen bonds: Electronegativity is the amount of pull that the members of a covalent bond exert on their shared electrons. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Web a molecule of water has two hydrogen atoms. Benzophenone reacts with sodium borohydride in the.Water
Properties of Water Presentation Biology
water Definition, Chemical Formula, Structure, Molecule, & Facts
PPT Water Chemistry & Properties of Water PowerPoint Presentation
Primary and Secondary Bonds Owlcation
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
The Unique Properties Of Water How Hydrogen Bonding Affects Our Body
Properties of Water Presentation Biology
Water Review
Hydrogen Bonds — Overview & Examples Expii
Related Post:

.PNG)




.PNG)