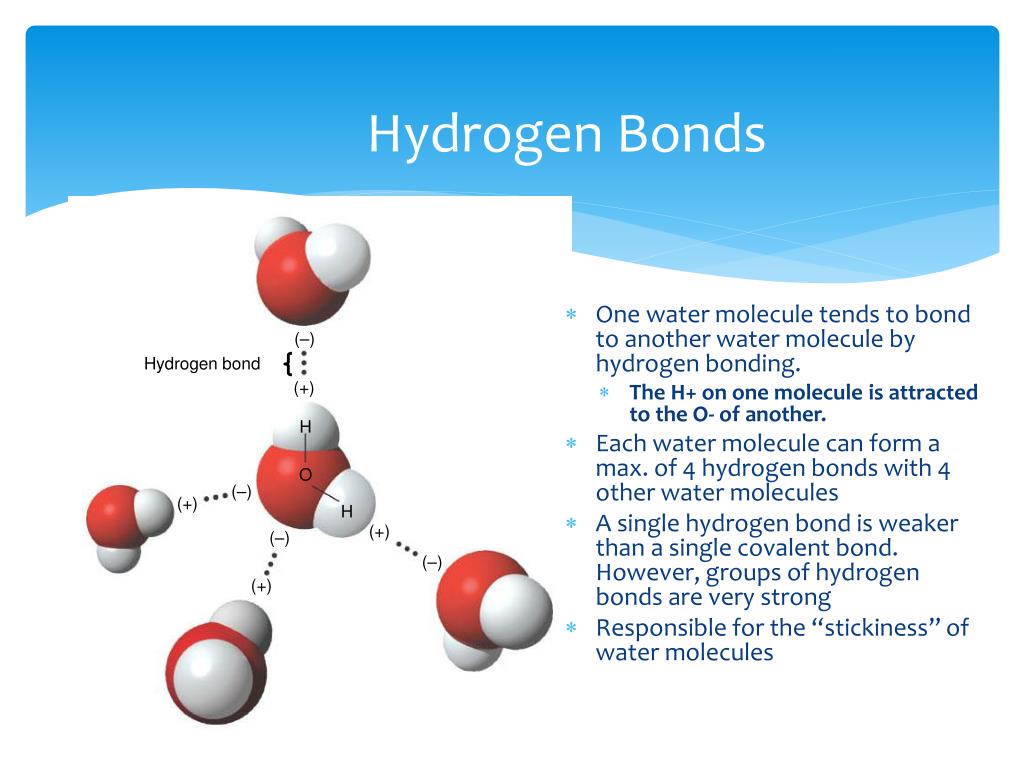
How Many Hydrogen Bonds Can A Single Water Molecule Form
How Many Hydrogen Bonds Can A Single Water Molecule Form - So yes, we can have hydrogen bonding. Assume all possible sites are available. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as. Answer all questions below 4. Web how many hydrogen bonds can a single water molecule form? Water is made up of two hydrogen atoms and an oxygen atom. A water molecule can form four hydrogen bonds. Two given through the h atoms (towards two other h2o molecules), and two received on the o atom (from. Web that molecule is involved in 4 hydrogen bonds. Covalent bonds are bonds that are formed when two atoms share their. Water is made up of two hydrogen atoms and an oxygen atom. Hydrogen bonds plants transport water to their leaves through the xylem when water evaporates from the. Web that molecule is involved in 4 hydrogen bonds. Hydrogen bonding is responsible for ammonia 's remarkably high solubility in. Web how many hydrogen bonds can a single water molecule form? Assume all possible sites are available. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. The oxygen atom can accept two. Web how many hydrogen bonds can form between a single ether molecule and water molecules? Hydrogen bonds plants transport water to their leaves through the xylem. Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Ad browse & discover thousands of science book titles, for less. Web how many hydrogen bonds can form between a single ether molecule and water molecules? Web we would like to show you a. So each water molecule can form a. The structure of water molecules and how they can interact to form hydrogen bonds. Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Assume all possible sites are available. The oxygen atom can accept two. Web that molecule is involved in 4 hydrogen bonds. Each water molecule is surrounded by four neighboring h 2 os. Web so far, we’ve drawn this water molecule with one hydrogen bond. Web water also has two lone pairs and two h atoms attached to the highly electronegative oxygen. Ad browse & discover thousands of science book titles, for less. The structure of water molecules and how they can interact to form hydrogen bonds. Another hydrogen bond can be formed using the other lone pair on the oxygen atom. Hydrogen bonds plants transport water to their leaves through the xylem when water evaporates from the. Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Web how many. The structure of water molecules and how they can interact to form hydrogen bonds. Web each one of the lone pairs and the two covalently bonded hydrogen atoms can form a single hydrogen bond either with other water molecules or another type of. So yes, we can have hydrogen bonding. Ho 7 08 3 05 nh₂ oh Answer all questions. Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Each hydrogen atom in the molecule can also form a hydrogen bond. Web how many hydrogen bonds can form between a single ether molecule and water molecules? Web the 4 possible hydrogen bonds formed with a water molecule in ice. Web how many hydrogen bonds can a single. Web water molecule can have/form a maximum of four hydrogen bonds: Web how many hydrogen bonds can a single water molecule form? Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Ad browse & discover thousands of science book titles, for less. The. Web the 4 possible hydrogen bonds formed with a water molecule in ice. Web so far, we’ve drawn this water molecule with one hydrogen bond. Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Covalent bonds are bonds that are formed when two. Each water molecule is surrounded by four neighboring h 2 os. Answer all questions below 4. But if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer. This means each water molecule can participate in up to 4. A water molecule can form four hydrogen bonds. Web so far, we’ve drawn this water molecule with one hydrogen bond. Web water molecule can have/form a maximum of four hydrogen bonds: Another hydrogen bond can be formed using the other lone pair on the oxygen atom. Water is made up of two hydrogen atoms and an oxygen atom. Web how many hydrogen bonds can a water molecule form? Web water also has two lone pairs and two h atoms attached to the highly electronegative oxygen. Polarity is the result of electronegativity. So each water molecule can form a. So yes, we can have hydrogen bonding. Hydrogen bonding is responsible for ammonia 's remarkably high solubility in. Hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Ad browse & discover thousands of science book titles, for less. Covalent bonds are bonds that are formed when two atoms share their. Ho 7 08 3 05 nh₂ oh How many hydrogen bonds can a single water molecule form?Hydrogen Bonds — Overview & Examples Expii
Water
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
water Definition, Chemical Formula, Structure, Molecule, & Facts
Water Review
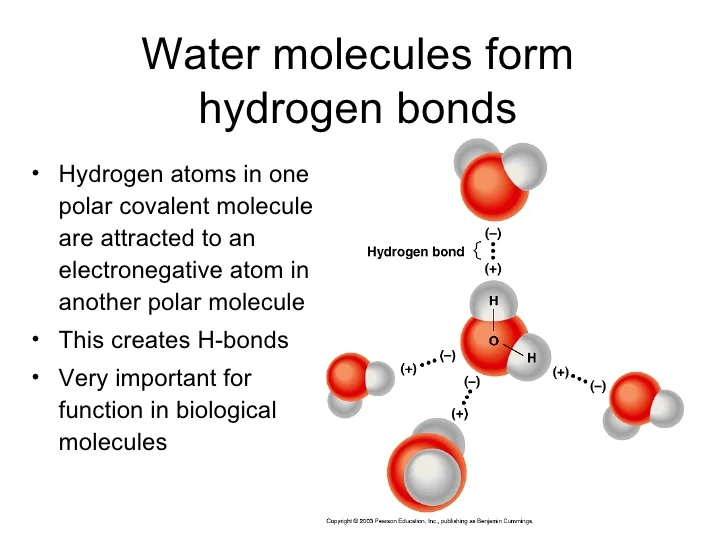
Water has both a hydrogen bond and a polar covalent bond. Hydrogen
Properties of Water Presentation Biology
Science online The importance of the water and its structure
Properties of Water Presentation Biology
PPT Properties of Water PowerPoint Presentation, free download ID
Related Post:





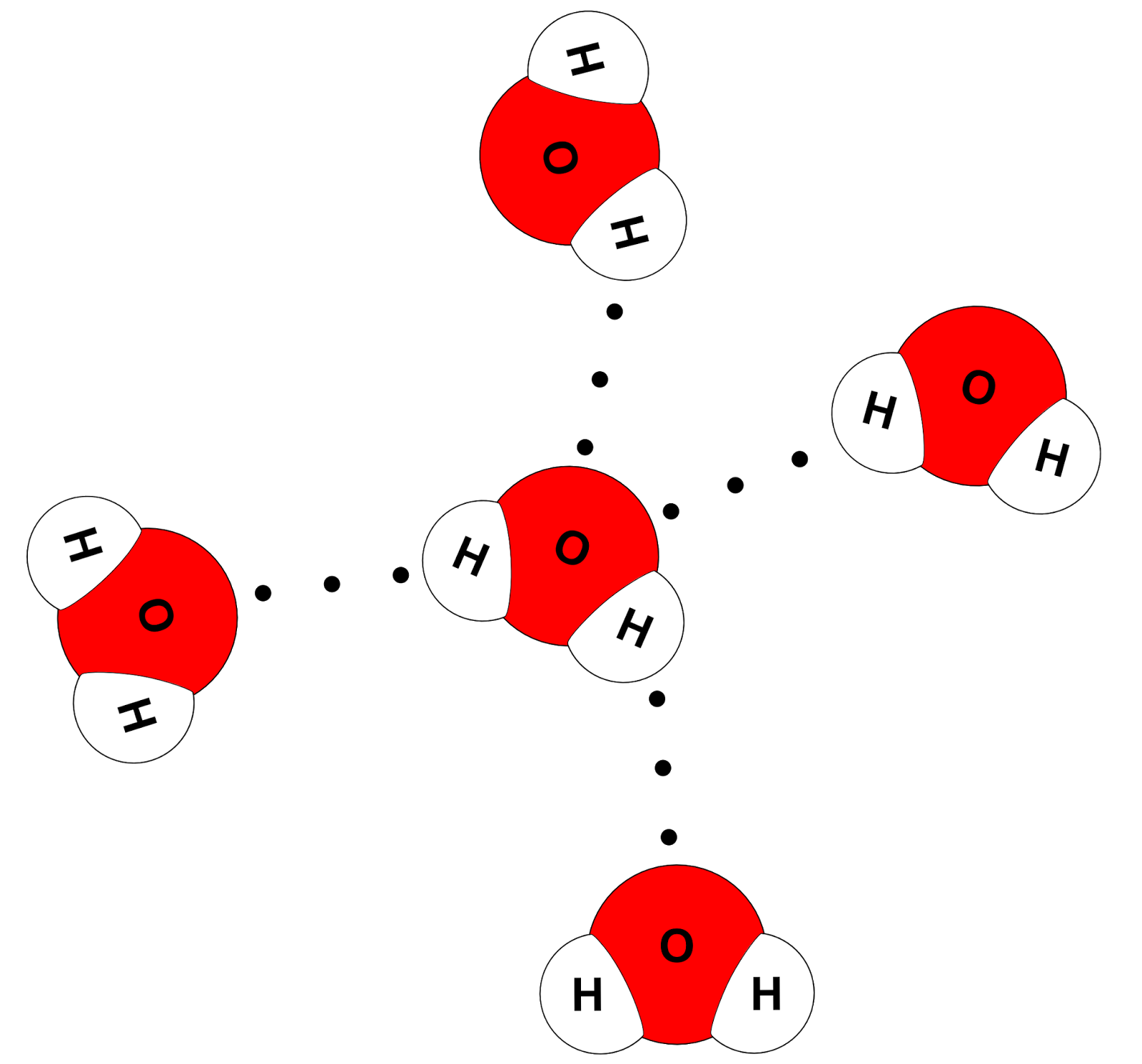
.PNG)
.PNG)