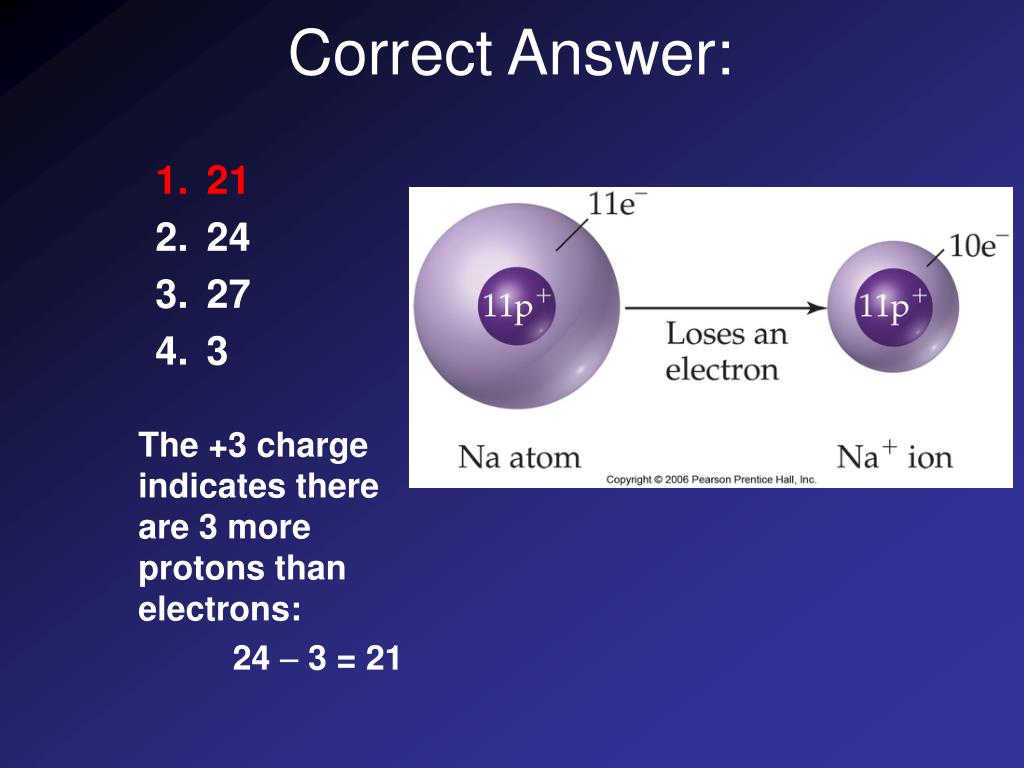
How Many Electrons Are Needed To Form A Charge Of
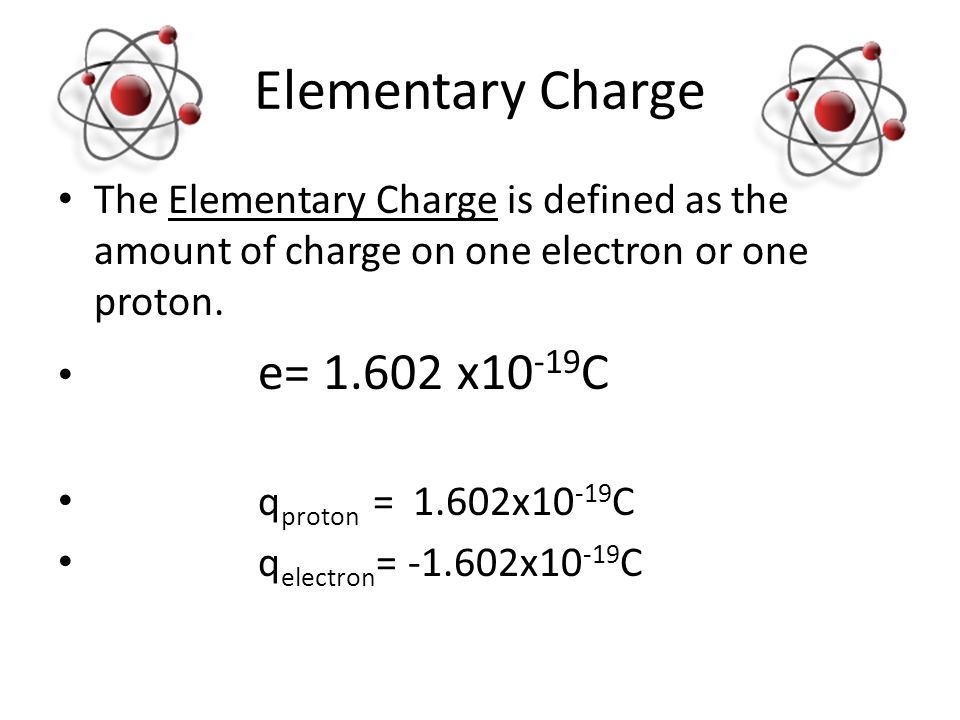
How Many Electrons Are Needed To Form A Charge Of - Thus, the charge on a proton is e ,. John’s school vs osei tutu shs vs opoku ware school Common static electricity involves charges ranging from nanocoulombs to microcoulombs. Since one electron has charge of q =. (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. Here, q represents the charge and e represents the charge. Web the same number of electrons is required to make −1.00 c of electric charge. (a) how many electrons are needed to form a charge. Web only one more electron is needed to achieve an octet in chlorine’s valence shell. (a) how many electrons are needed to form a charge. Web common static electricity involves charges ranging from nanocoulombs to microcoulombs. Web so, let’s apply our equation: N= sin () cos) tan) cotan asino acos ) atan () acotan) sinh cosho) tanh (). Electrons have a charge of − 1.602 × 10 − 19 c. The fundamental unit of charge is often represented as e. Become a study.com member to unlock this answer! Our final result indicates that a calcium ion (ca^2+). Web electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal to 1.602176634 × 10−19 coulomb. Here, q represents the charge and e represents the charge. This problem has been solved! Web normally two electrons pairs up and forms a bond, e.g., \(h_2\) for most atoms there will be a maximum of eight electrons in the valence shell (octet structure),. Web so, let’s apply our equation: The fundamental unit of charge is often represented as e. Web common static electricity involves charges ranging from nanocoulombs to microcoulombs. N= part (b) how. Web (a) how many electrons are needed to form a charge of −2.00 nc? Become a study.com member to unlock this answer! Terms in this set (14) 3.1. N= part (b) how many. The fundamental unit of charge is often represented as e. (a) how many electrons are needed to form a charge. Web up to 24% cash back solution. Common static electricity involves charges ranging from nanocoulombs to microcoulombs. Web electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal to 1.602176634 × 10−19 coulomb. N= part (b) how many. (a) how many electrons are needed to form a charge. Common static electricity involves charges ranging from nanocoulombs to microcoulombs. Web up to 24% cash back solution. (b) how many electrons must be removed from a neutral object to leave a net charge of 0.500 \mu. This problem has been solved! Web electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal to 1.602176634 × 10−19 coulomb. You'll get a detailed solution from a subject matter expert that helps you learn. Web common static electricity involves charges ranging from nanocoulombs to microcoulombs. (a) how many electrons are needed to form a charge. This problem has. Web normally two electrons pairs up and forms a bond, e.g., \(h_2\) for most atoms there will be a maximum of eight electrons in the valence shell (octet structure),. Our final result indicates that a calcium ion (ca^2+). Web how many electrons are needed to make a total charge of 1c? N= sin () cos) tan) cotan asino acos ). Common static electricity involves charges ranging from nanocoulombs to microcoulombs. Here, q represents the charge and e represents the charge. Terms in this set (14) 3.1. A numeric value is expected and not an expression. Web so, let’s apply our equation: Web how many electrons are needed to form a charge of −6.90 nc? N= part (b) how many. (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. Since one electron has charge of q =. A numeric value is expected and not an expression. This problem has been solved! Web so, let’s apply our equation: Thus, the charge on a proton is e ,. N= part (b) how many. Web the tiny superscripts say how many electrons live in each orbital, the letters represent the orbitals that are available, and the big numbers say which energy level the orbitals are. N= sin () cos) tan) cotan asino acos ) atan () acotan) sinh cosho) tanh (). (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. How many electrons must be removed from a neutral object to leave a net charge of q 2 = 2.4 μc? The required number of electrons can be determined by the following equation: (b) how many electrons must be removed from a neutral object to leave a net charge of 0.500 \mu. Web up to 24% cash back solution. (a) how many electrons are needed to form a charge. A numeric value is expected and not an expression. Web electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal to 1.602176634 × 10−19 coulomb. Web how many electrons are needed to form a charge of −6.90 nc? Web physics questions and answers. Web only one more electron is needed to achieve an octet in chlorine’s valence shell. Terms in this set (14) 3.1. Web the same number of electrons is required to make −1.00 c of electric charge. John’s school vs osei tutu shs vs opoku ware schoolPPT Atoms, Molecules & Ions PowerPoint Presentation, free download
How many electrons are there in 1 Coulomb of charge? Teachoo
calculate the number of electrons in 10uc of charge Science
tps 50 Part (a) How many electrons are needed to form a charge of Q1
What are the Difference Between Charge and Electron?
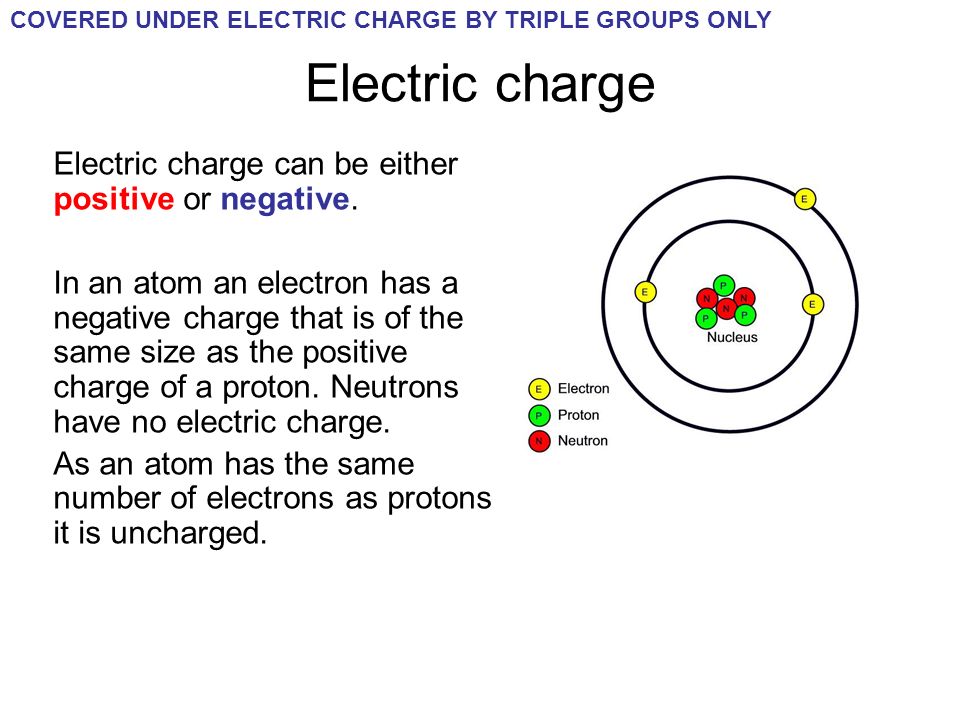
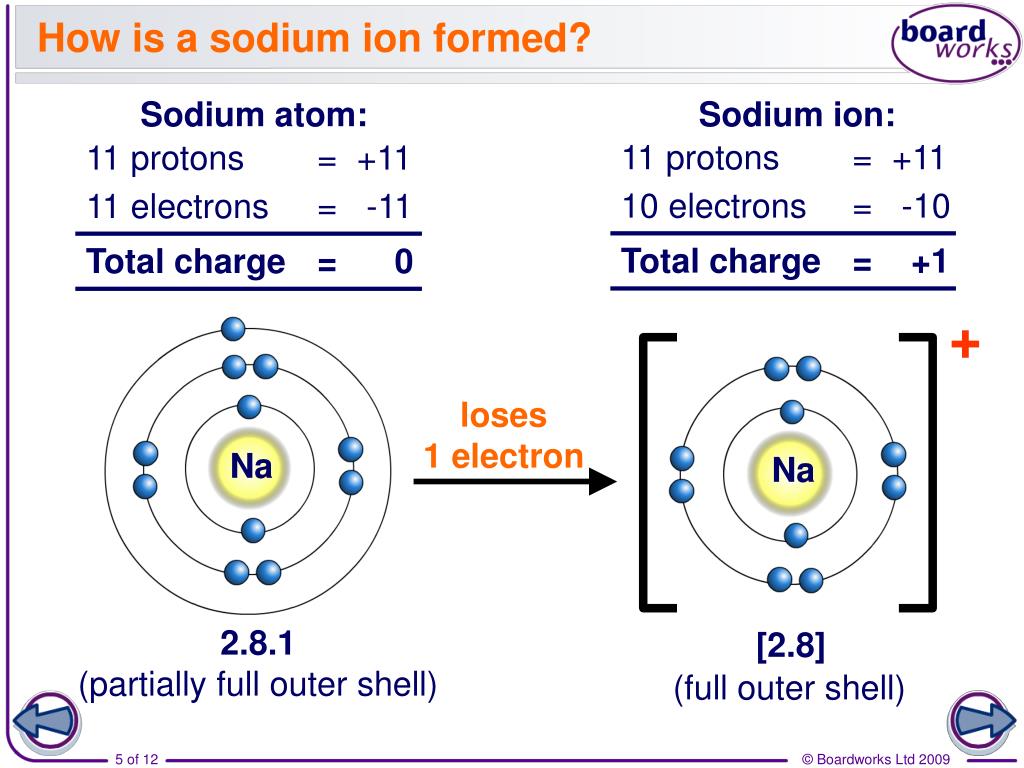
PPT How do atoms form ions? PowerPoint Presentation ID7021047
What are the Difference Between Charge and Electron?
How many electrons are required to make a charge of 50 micro coulombs
Draw A Simple Diagram Of An Atom Labeled Protons Neutrons And Electrons
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint
Related Post: