How Many Covalent Bonds Can Silicon Form
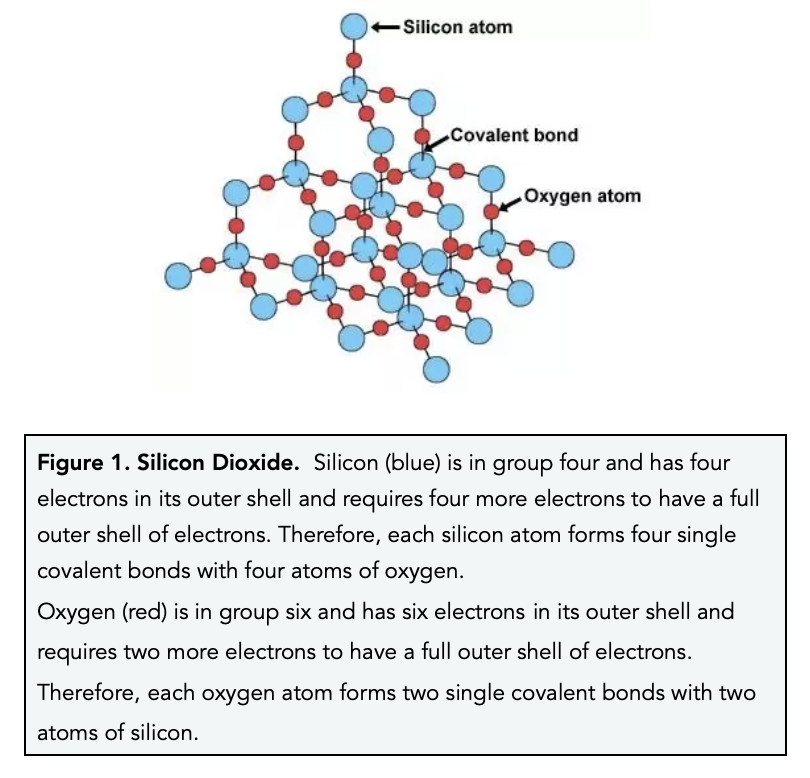
How Many Covalent Bonds Can Silicon Form - The element silicon would be expected to form 4 covalent bond(s) in order to obey the octet rule. Some plants accumulate silica in their tissues and require silicon for their growth, for example rice. These simplified diagrams do not do justice to the nature of that. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web boron forms three covalent bonds with silicon, leaving one silicon atom frustrated, not forming a bond. Web covalent network solids include crystals of diamond, silicon, some other nonmetals, and some covalent compounds such as silicon dioxide (sand) and silicon carbide. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. An introduction to physical science. Is determined by the distance at which the lowest potential energy is achieved. The potential energy of two separate hydrogen atoms (right) decreases as. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Diatoms, radiolaria, and siliceous sponges use biogenic silica as a structural material for their skeletons. Web the number of electrons required to obtain an octet determines the number of covalent bonds an. The atoms are joined to each other in a regular. Web the element silicon would be expected to form 4 covalent bond (s) in order to obey the octet rule. An introduction to physical science. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. This doping process introduces the idea of. This doping process introduces the idea of the hole, that. Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule and only. An introduction to physical science. Meallic elements can definiely have more than eight. Silicon may be taken up by plants as orthosilicic acid (also known as monosilicic acid) and. The carbon atom is unique among elements in its tendency to form extensive networks of covalent. Web boron forms three covalent bonds with silicon, leaving one silicon atom frustrated, not forming a bond. Is determined by the distance at which the lowest potential energy is achieved. Web for that same reason, six or seven bonds are possible, and xenon can. The element silicon would be expected to form 4 covalent bond(s) in order to obey the octet rule. Web boron forms three covalent bonds with silicon, leaving one silicon atom frustrated, not forming a bond. This is summarized in the table below. Web the main point here is that a silicon atom has four electrons which it can share in. Meallic elements can definiely have more than eight. An introduction to physical science. Is determined by the distance at which the lowest potential energy is achieved. The carbon atom is unique among elements in its tendency to form extensive networks of covalent. There is a quick way to work out how many covalent bonds an element. The major (antisymmetric) component involves the radial. Web how many covalent bonds are formed by silicon? Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule and only. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web boron forms. Web for that same reason, six or seven bonds are possible, and xenon can form 8 covalent bonds in the compound xeo4! It contains many silicon and oxygen atoms. Some plants accumulate silica in their tissues and require silicon for their growth, for example rice. Silicon may be taken up by plants as orthosilicic acid (also known as monosilicic acid). An introduction to physical science. 1.in silicon crystal the outer most class has 4 electrons.these 4 electrons are ready to pair to ge. It contains many silicon and oxygen atoms. Web so in order to be stable, silicon$ (si)$needs to form four covalent bonds. Is determined by the distance at which the lowest potential energy is achieved. Silicon dioxide, takes on this molecular form, instead of carbon dioxide's characteristic shape, because silicon's 3p orbitals make it more energetically. Web boron forms three covalent bonds with silicon, leaving one silicon atom frustrated, not forming a bond. * it forms four bon. Web the element silicon would be expected to form 4 covalent bond (s) in order to obey. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. The atoms are joined to each other in a regular. This is summarized in the table below. Web the element silicon would be expected to form 4 covalent bond (s) in order to obey the octet rule. Silicon dioxide, takes on this molecular form, instead of carbon dioxide's characteristic shape, because silicon's 3p orbitals make it more energetically. Although silicon is readily available in the form of silicates, very few organisms use it directly. Meallic elements can definiely have more than eight. Silicon may be taken up by plants as orthosilicic acid (also known as monosilicic acid) and tran… Web boron forms three covalent bonds with silicon, leaving one silicon atom frustrated, not forming a bond. These simplified diagrams do not do justice to the nature of that. The potential energy of two separate hydrogen atoms (right) decreases as. There is a quick way to work out how many covalent bonds an element. Web covalent network solids include crystals of diamond, silicon, some other nonmetals, and some covalent compounds such as silicon dioxide (sand) and silicon carbide. * it forms four bon. 1.in silicon crystal the outer most class has 4 electrons.these 4 electrons are ready to pair to ge. Some plants accumulate silica in their tissues and require silicon for their growth, for example rice. Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule and only. Web the main point here is that a silicon atom has four electrons which it can share in covalent bonds with its neighbors. Web (1) silicon (si) * it usually forms four bonds with all compounds except fluorine. Web as a group 14 element, each silicon atom has four valence electrons.2.2 Bonding and Lattices Physical Geology
Schematic representation of covalent bonds in a silicon crystal lattice
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind
Semiconductor Basics Tutorial Electrical Academia
PN Junction Formation & Structure Electrical Academia
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
How Many Single Covalent Bonds Must A Silicon Atom Form
Pure silicon consists of atoms that form covalent bonds with adjacent
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
How Many Single Covalent Bonds Must A Silicon Atom Form
Related Post: