How Many Covalent Bonds Can Each Carbon Atom Form
How Many Covalent Bonds Can Each Carbon Atom Form - The potential energy of two separate hydrogen atoms (right) decreases as. This means that it has six protons and six electrons. Web carbon can form four covalent bonds to create an organic molecule. Web covalent bonds involve the sharing of electron pairs between atoms. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. Group 5a form 3 bonds; Web typically, the atoms of group 4a form 4 covalent bonds; Web for example, each atom of a group 4a element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Web typically, the atoms of group 4a form 4 covalent bonds; With hydrogen, nitrogen, oxygen, and other heteroatoms. The atomic number of carbon is 6. Web 4 rows for example, each atom of a group 14 element has four electrons in its outermost shell and. The potential energy of two separate hydrogen atoms (right) decreases as. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. It has the chemical formula. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. The number of electrons required to. Web for example, each atom of a group 4a. Group 5a form 3 bonds; Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Web carbon can form four covalent bonds to create an organic molecule. Web typically, the atoms of group 4a form 4 covalent bonds; The potential energy of two separate hydrogen atoms (right) decreases as. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. There is a quick way to work out how many covalent bonds an element. With hydrogen, nitrogen, oxygen, and other heteroatoms. The methane molecule provides an example: It has the chemical formula. Web covalent bonds involve the sharing of electron pairs between atoms. The potential energy of two separate hydrogen atoms (right) decreases as. The methane molecule provides an example: The simplest carbon molecule is methane (ch 4 ), depicted here. Web 4 rows for example, each atom of a group 14 element has four electrons in its outermost shell and. Web covalent bonds involve the sharing of electron pairs between atoms. Web 4 rows typically, the atoms of group 4a form 4 covalent bonds; These four electrons can be. Web for example, each atom of a group 4a element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. The atomic number of. This means that it has six protons and six electrons. Web for example, each atom of a group 4a element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. And group 7a form one bond. It has the chemical formula. Group 5a form 3 bonds; Group 5a form 3 bonds; Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. The simplest carbon molecule is methane (ch 4 ), depicted here. The atomic number of carbon is 6. And group 7a form one bond. Well, carbon can form up to four covalent bonds. These four electrons can be. Group 5a form 3 bonds; Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Web typically, the atoms of group 4a form 4 covalent bonds; Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Web 4 rows typically, the atoms of group 4a form 4 covalent bonds; Web typically, the atoms of group 4a form 4 covalent bonds; Web covalent bonds involve the sharing of electron pairs between atoms. Web moreover, of all the elements in the second row,. Web covalent bonds involve the sharing of electron pairs between atoms. Web carbon can form four covalent bonds to create an organic molecule. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. These four electrons can be. And group 7a form one bond. Web 4 rows for example, each atom of a group 14 element has four electrons in its outermost shell and. The potential energy of two separate hydrogen atoms (right) decreases as. Group 5a form 3 bonds; 4 alkanes are hydrocarbons that contain what type of bonds? A bond composed of two electrons , one from each of. There is a quick way to work out how many covalent bonds an element. Web typically, the atoms of group 4a form 4 covalent bonds; It has the chemical formula. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. With hydrogen, nitrogen, oxygen, and other heteroatoms. When it bonds only with hydrogen, it forms compounds called. The simplest carbon molecule is methane (ch 4 ), depicted here. Is determined by the distance at which the lowest potential energy is achieved. Web typically, the atoms of group 4a form 4 covalent bonds;hillis2e_ch02
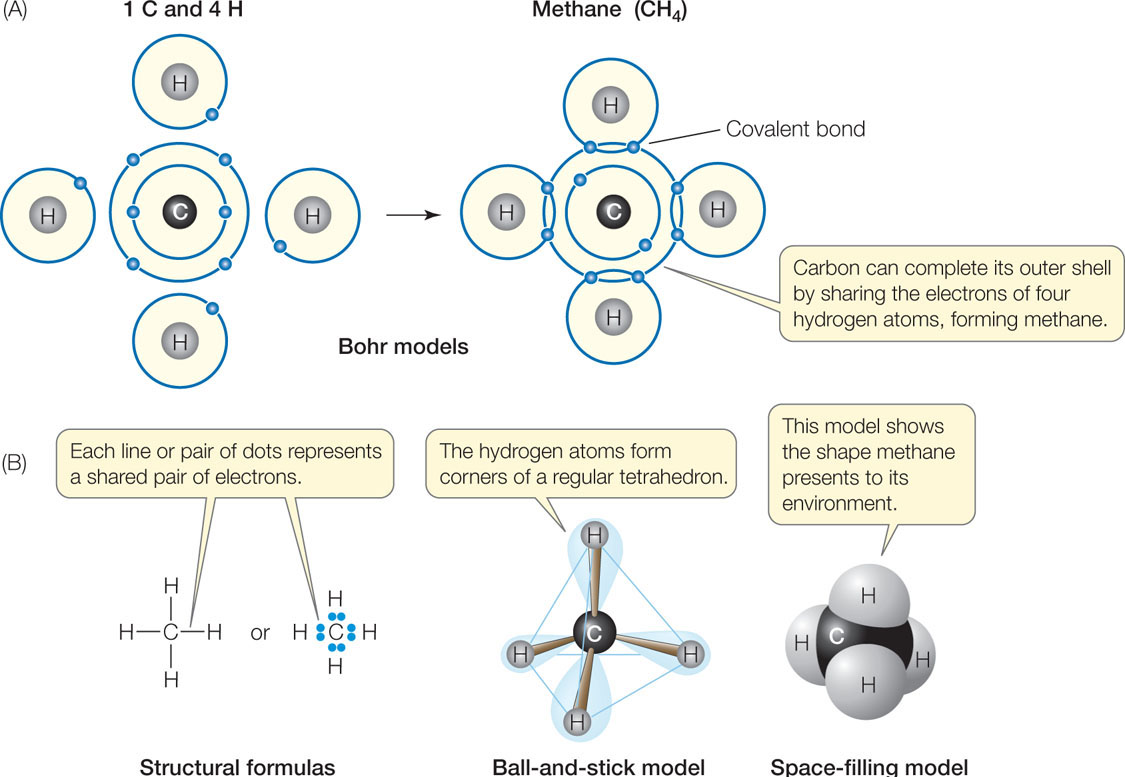
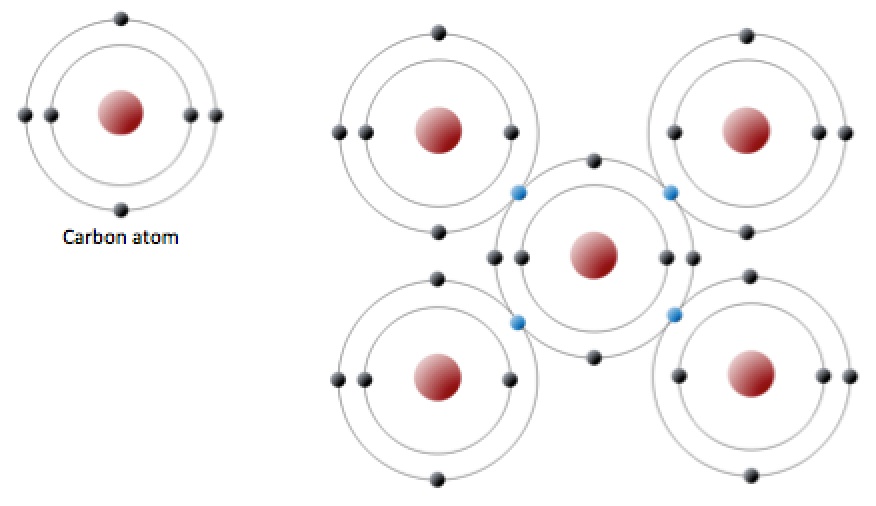
Four covalent bonds. Carbon has four valence electrons and here a
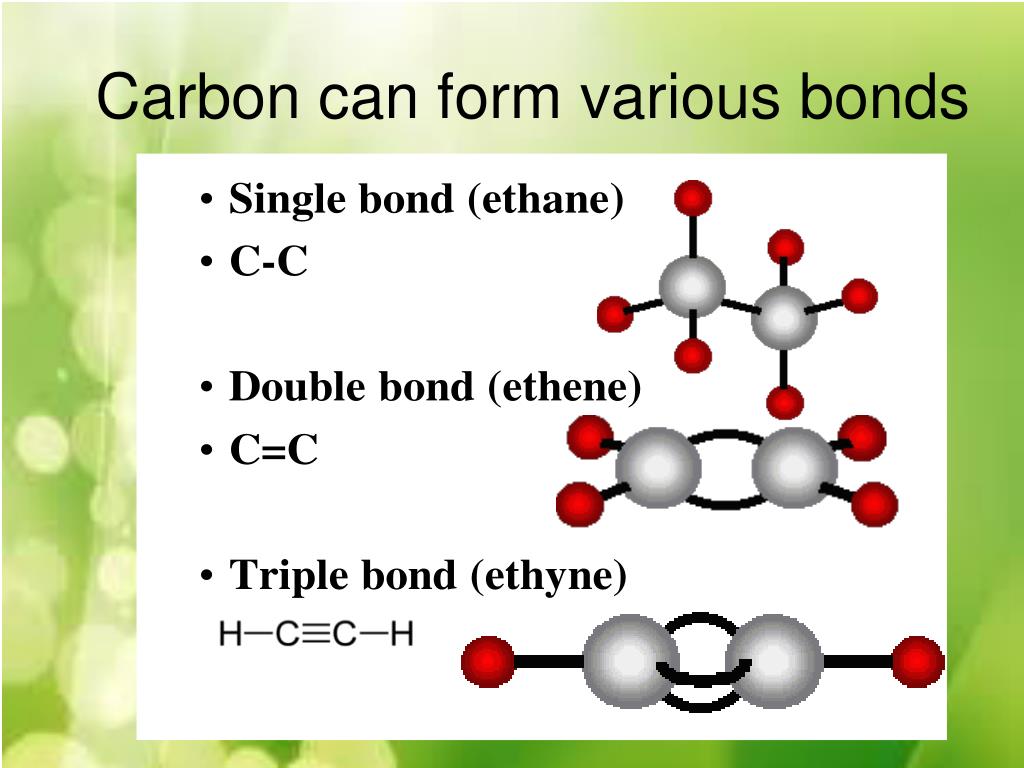
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
chemistry picture
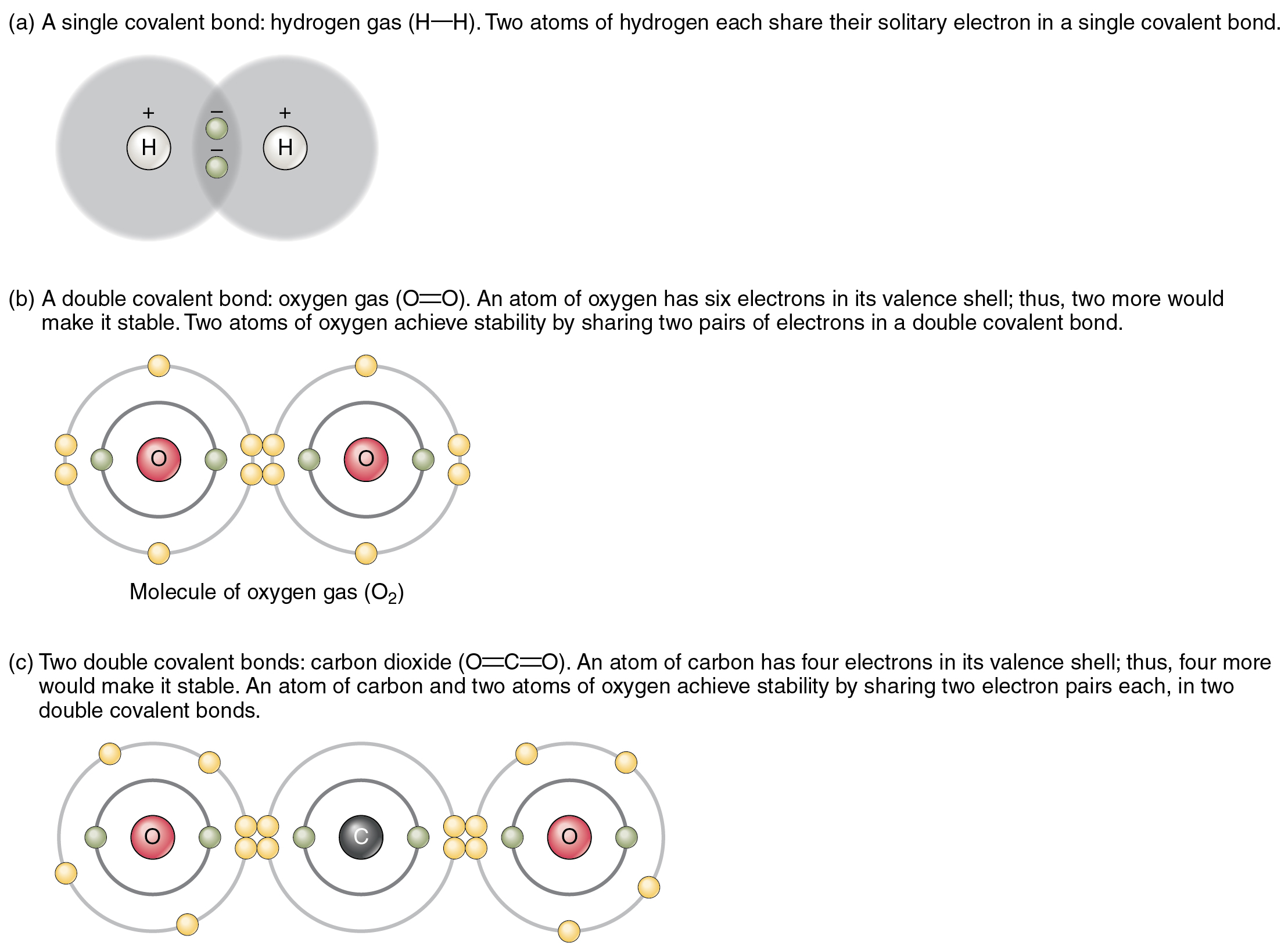
The top panel in this figure shows two hydrogen atoms sharing two
Double Covalent Bond Facts, Definition, History & Examples
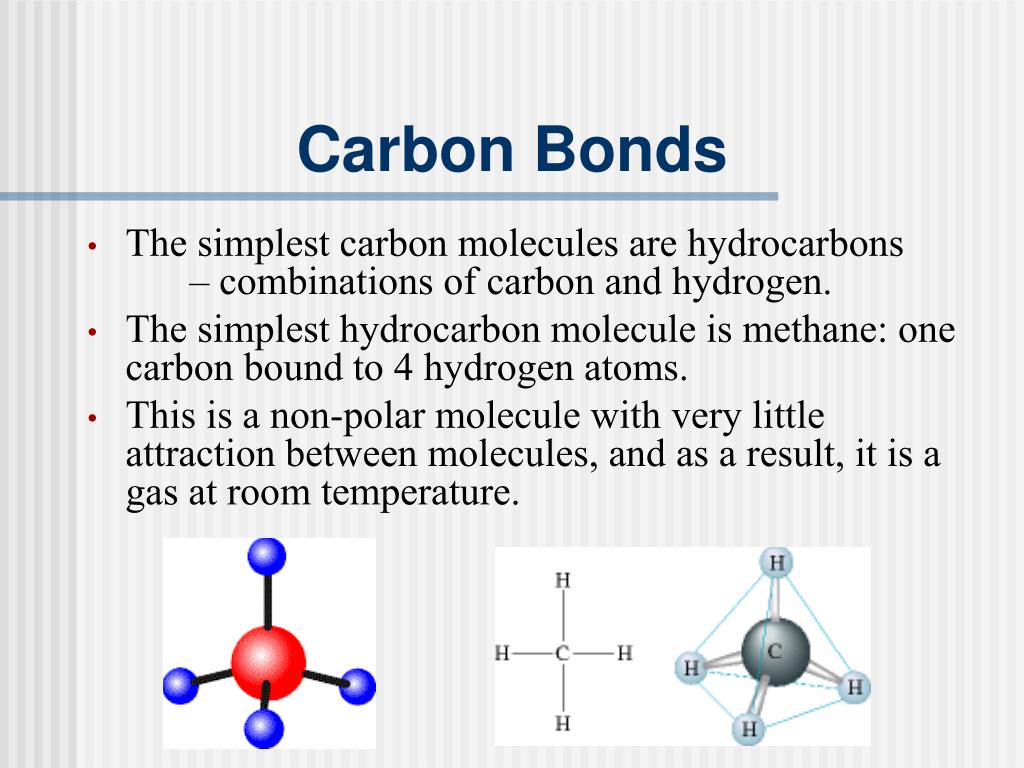
How many covalent bonds does carbon form if each of its unpaired
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
PPT Biochemistry PowerPoint Presentation, free download ID89333
2.2 Bonding and Lattices Physical Geology
Related Post: