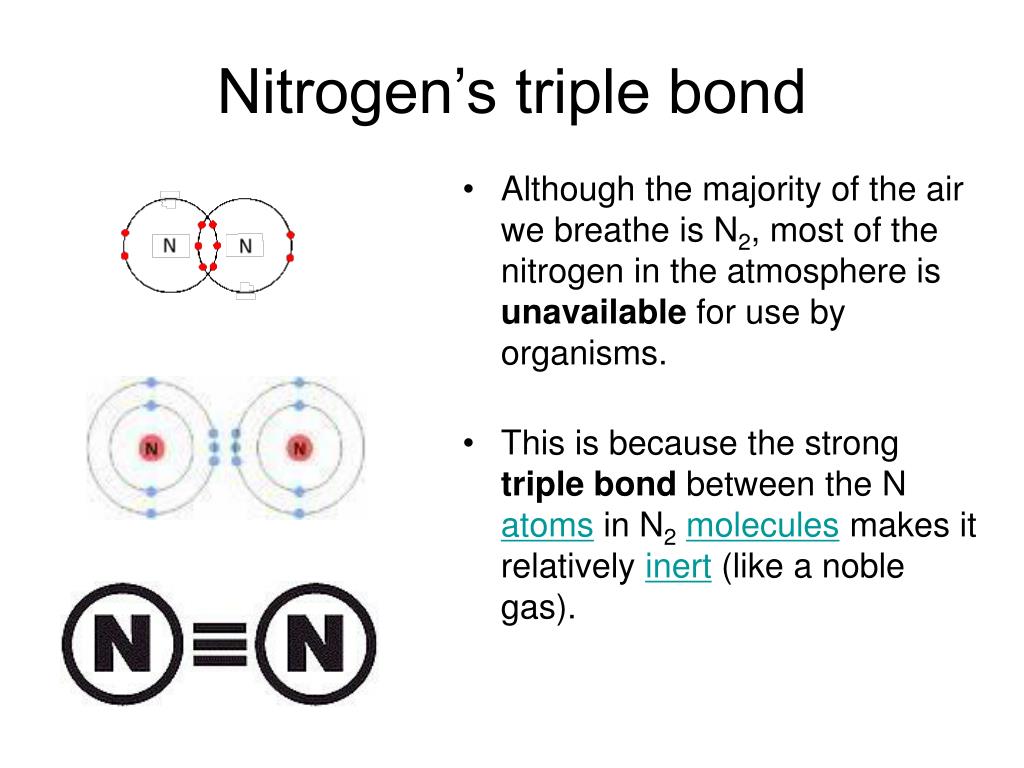
How Many Bonds Does Nitrogen Form
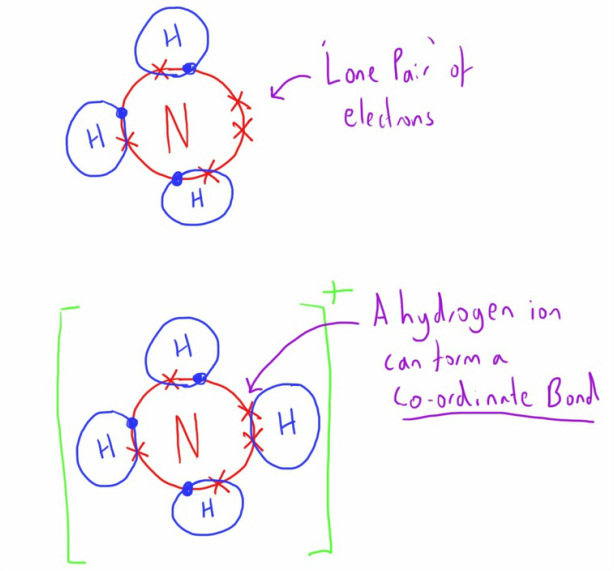
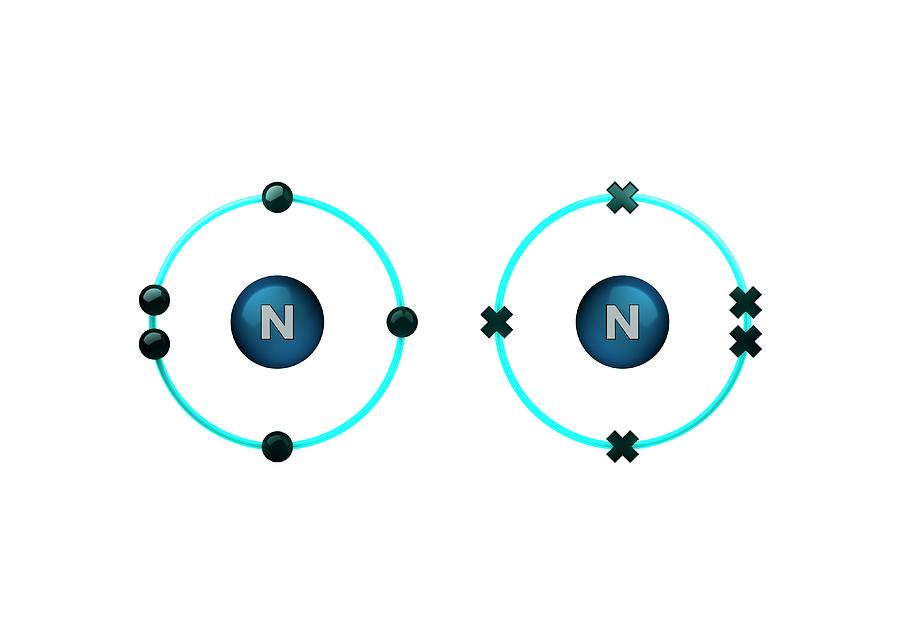
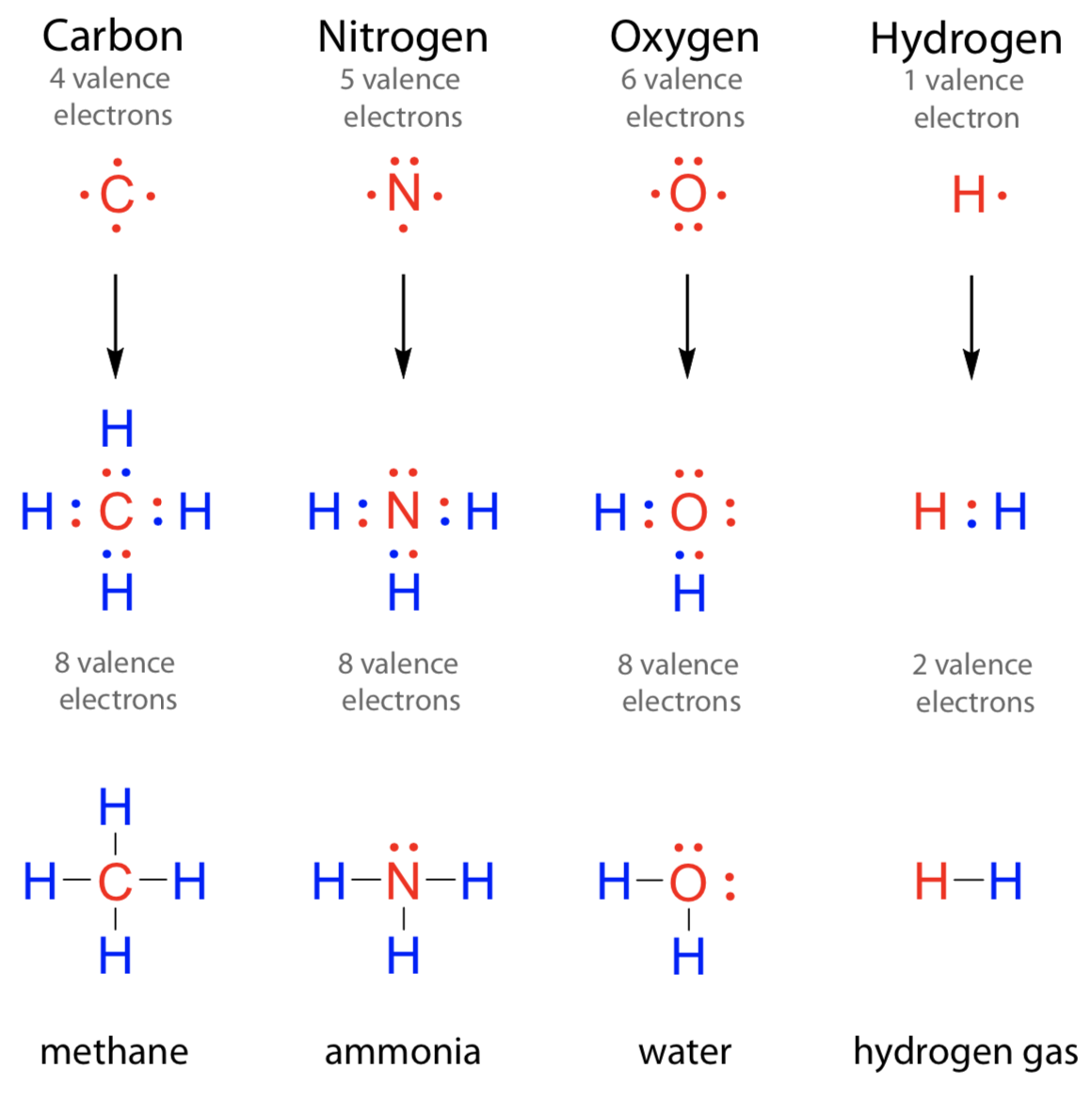
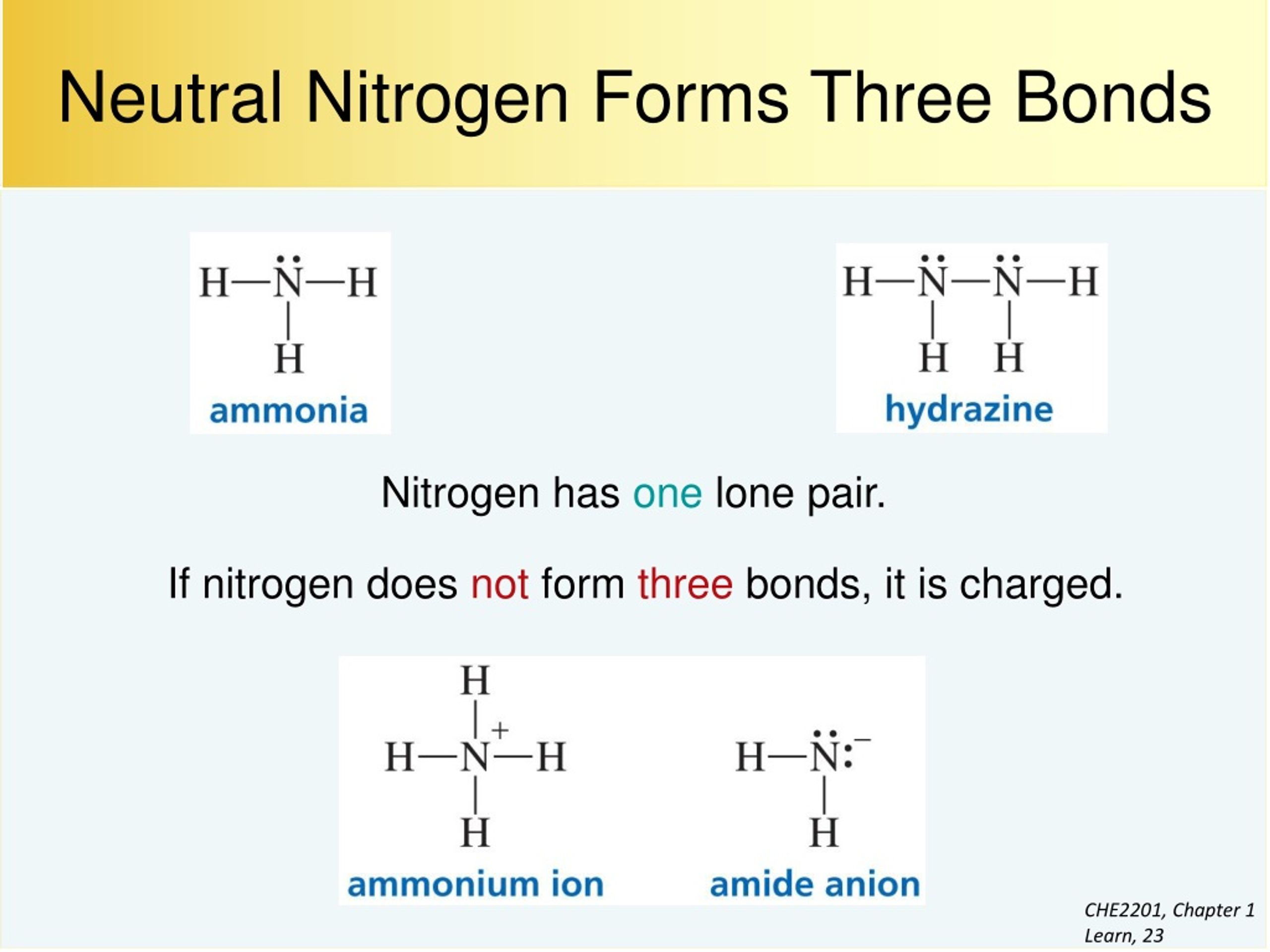
How Many Bonds Does Nitrogen Form - This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5. Group 5a form 3 bonds; We will also discuss the Group 6a form 2 bonds; This type of bond is very strong, which makes it useful in applications like explosives. Through that pair, nitrogen can form an. These are normally formed with other metals and look like: Therefore, it can form three bonds by sharing its three electrons. Members of a group typically have similar properties and electron configurations in their outer shell. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. Nitrogen can also form a double bond, in which. To obtain an octet, these atoms. It cannot accept any more electrons but here's how. Does this make sense based on the number of valence electrons in a nitrogen atom?. Ad browse & discover thousands of science book titles, for less. Web nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. But when we look carefully, we never. So when nitrogen has two bonds and. Web two different atoms can also share electrons and form covalent bonds. Web two different atoms can also share electrons and form covalent bonds. Web nitrogen forms 3 bonds. This type of bond is very strong, which makes it useful in applications like explosives. A vertical column in the periodic table. Nitrogen (group 15 or 5a) 3: Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three. To obtain an octet, these atoms. Web two different atoms can also share electrons and form covalent bonds. One lone pair and three unpaired electrons. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two hydrogen atoms. Web so if you are following the rules, you might well assume that nitrogen would be able to form five bonds (after all, it has five valence electrons). To obtain an octet, these atoms. But when we look carefully, we never. Web nitrogen. Members of a group typically have similar properties and electron configurations in their outer shell. Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds. This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5. To obtain an octet, these atoms. Ad browse & discover thousands of science book titles,. This type of bond is very strong, which makes it useful in applications like explosives. Therefore, it can form three bonds by sharing its three electrons. Members of a group typically have similar properties and electron configurations in their outer shell. In nitrogen contains fire valence electrons but it shows four coval.view the full answer Web nitrogen is a very. And group 7a form one bond. How many bonds does nitrogen typically form? These are normally formed with other metals and look like: Web typically, the atoms of group 4a form 4 covalent bonds; Web two different atoms can also share electrons and form covalent bonds. But when we look carefully, we never. Group 5a form 3 bonds; Group 6a form 2 bonds; Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds. Nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. Mn, m 3 n, and m 4. Web two different atoms can also share electrons and form covalent bonds. Web nitrogen is a very interesting element and in this blog post, we. Ad browse & discover thousands of science book titles, for less. A vertical column in the periodic table. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three. So when nitrogen has two bonds and. There is a quick way to work out how many covalent bonds an element will. One lone pair and three unpaired electrons. This is because it has atomic number 7, so its electron configuration is 1s22s22p3, giving it 5. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two hydrogen atoms. One lone pair and three unpaired electrons. To obtain an octet, these atoms. And group 7a form one bond. Nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. One lone pair and three unpaired electrons. Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Web typically, the atoms of group 4a form 4 covalent bonds; Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Members of a group typically have similar properties and electron configurations in their outer shell. It cannot accept any more electrons but here's how.image
PPT Organic compounds containing nitrogen and sulfur PowerPoint
Bond Formation In Nitrogen Molecule Photograph by
LabXchange
Nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID899587
How many bonds does Nitrogen form? YouTube
[Solved] How many bonds can nitrogen form? 9to5Science
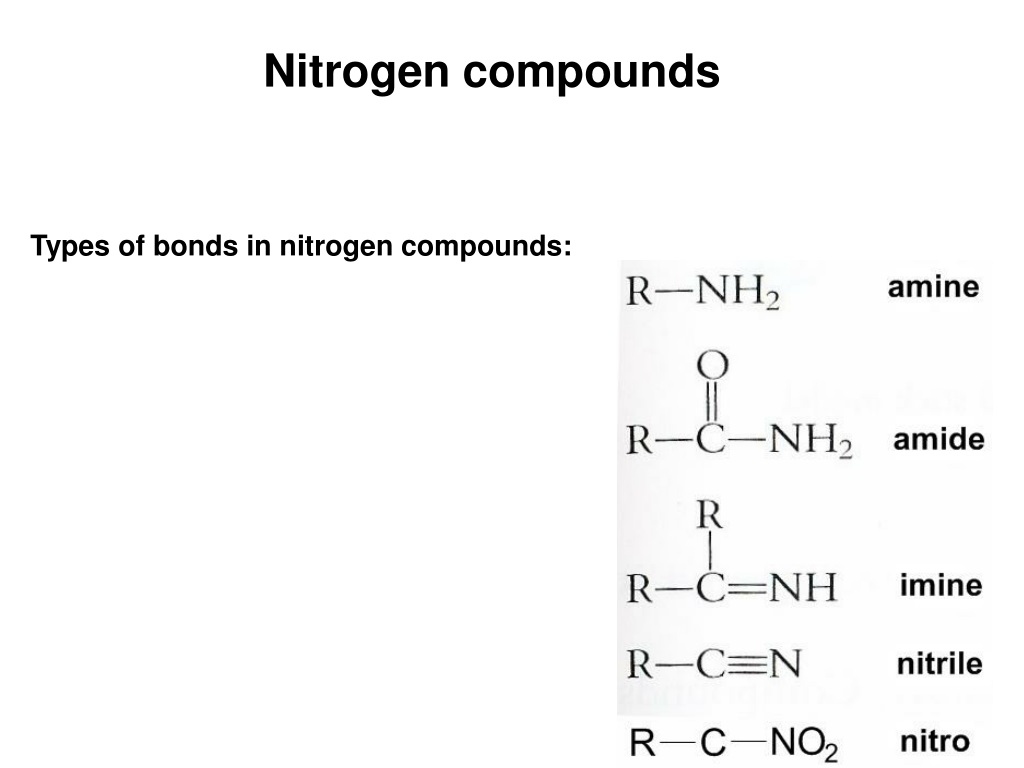
Grade 12 CHAPTER 4 NITROGEN COMPOUNDS. SEMESTER 1
PPT Structure and Bonding PowerPoint Presentation, free download ID
Related Post: