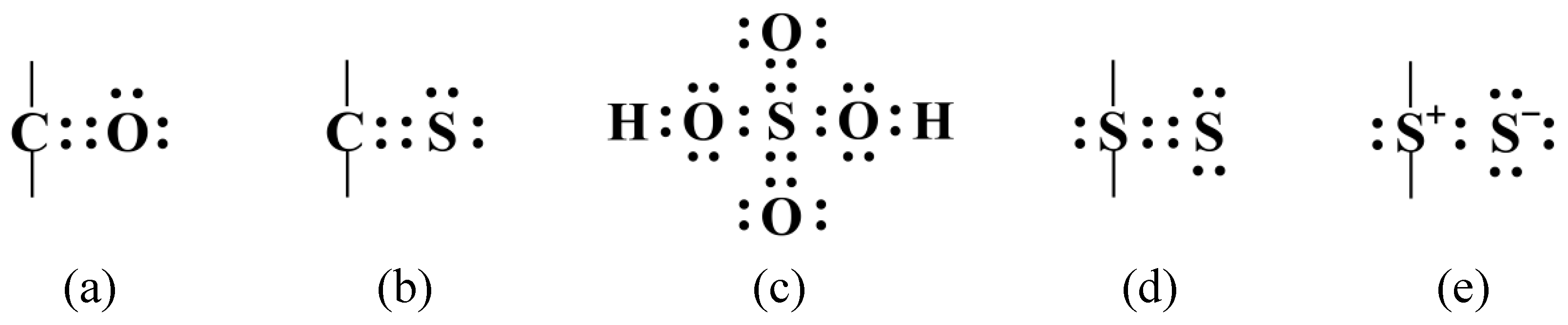
How Many Bonds Can Sulfur Form
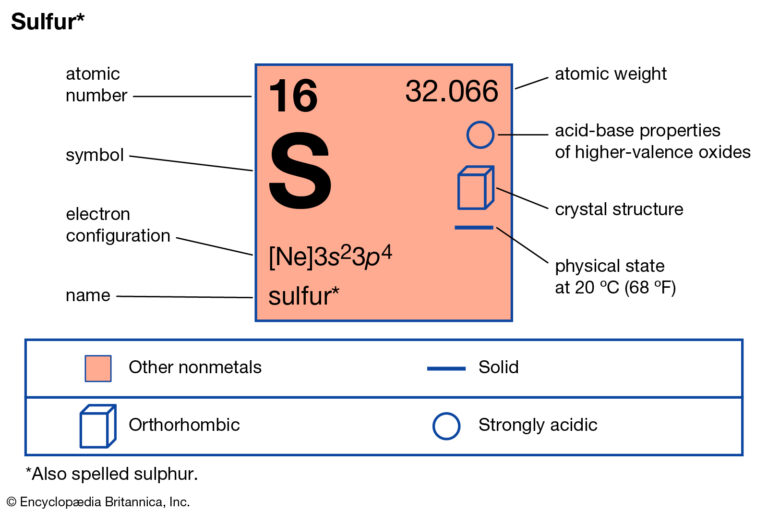
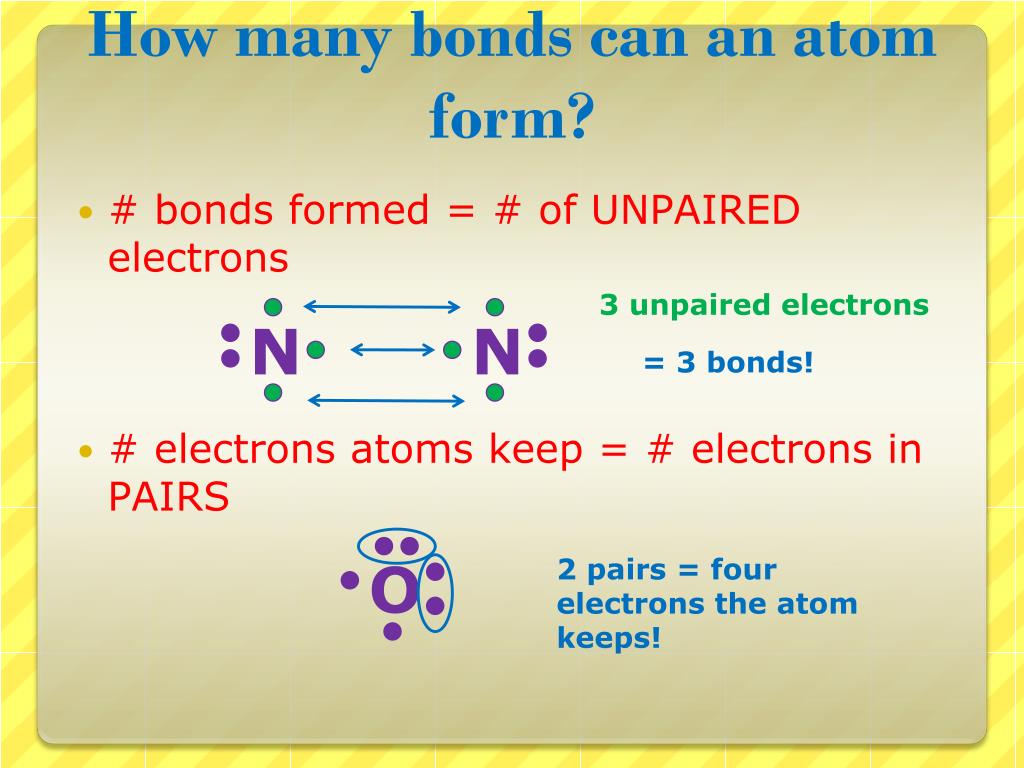
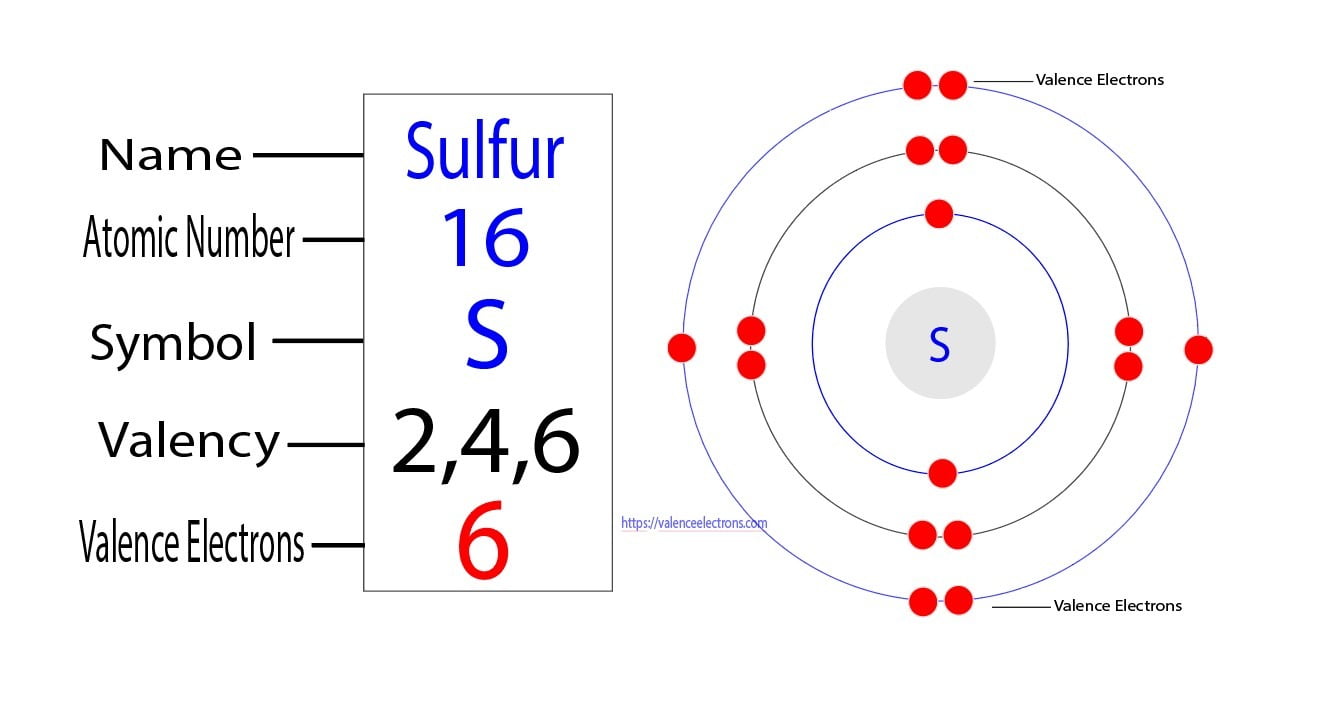
How Many Bonds Can Sulfur Form - Web answer and explanation: Web sulphur has electronic configuration : Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected. Ad browse & discover thousands of science book titles, for less. Sulfur is in period 3 of the periodic table. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its outermost. Web sulfur indeed can make up to 6 bonds. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web how many bonds can sulfur form? Ad browse & discover thousands of science book titles, for less. In fact, it is a multivalent atom, meaning it can make different number of bonds, up to 6, depending on the chemical formula. It can do this by forming. The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â. It is the 5 the most abundant element on earth. Web sulfur, nonmetallic chemical element, one of the most reactive of the elements. Sulfur is in period 3 of the periodic table. Sulfur is in period 3 of the periodic table. In fact, it is a multivalent atom, meaning it can make different number of bonds, up to 6, depending. Web sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a. It is the 5 the most abundant element on earth. Sulfur is in period 3 of the periodic table. In fact, it is a multivalent atom, meaning it can. Web study with quizlet and memorize flashcards containing terms like if sulfur has an atomic number of 16, how many covalent bonds can it form with other atoms? How many bonds can a sulfur atom form? The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected.. It can do this by forming. Sulfur can make 2, 4 or 6 bonds. Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a. It is the 5 the most abundant element on earth. Number of bonds an oxygen atom often forms in a covalent compound. Sulfur can make 2, 4 or 6 bonds. Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected. Web sulfur is capable of forming 6 bonds because it can have an. Number of bonds an oxygen atom often forms in a covalent compound. How many bonds can a sulfur atom form? Sulfur is in period 3 of the periodic table. Web up to 6% cash back sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons. Web study with quizlet and memorize flashcards containing terms like if. The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected. Web terms in this set (29) number of bonds a nitrogen atom often forms in a covalent compound. Sulfur is in period 3 of the periodic table. Sulfur can make 2, 4 or 6 bonds. Web. Web sulphur has electronic configuration : Sulfur is in period 3 of the periodic table. Sulfur is in period 3 of the periodic table. It can do this by forming. In fact, it is a multivalent atom, meaning it can make different number of bonds, up to 6, depending on the chemical formula. Number of bonds an oxygen atom often forms in a covalent compound. The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected. In fact, it is a multivalent atom, meaning it can make different number of bonds, up to 6, depending on the chemical formula. Pure. Web study with quizlet and memorize flashcards containing terms like if sulfur has an atomic number of 16, how many covalent bonds can it form with other atoms? Web sulphur has electronic configuration : Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Pure sulfur is a tasteless, odorless, brittle solid that is pale yellow in color, a. Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. Sulfur is in period 3 of the periodic table. Examples of compounds with these valencies for sulfur are iron (ii) sulfide, fes, sulfurous acid,. How many bonds can a sulfur atom form? The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its outermost. Web sulfur indeed can make up to 6 bonds. Web sulfur, nonmetallic chemical element, one of the most reactive of the elements. It is the 5 the most abundant element on earth. Web answer and explanation: Sulfur is in period 3 of the periodic table. Number of bonds an oxygen atom often forms in a covalent compound. Sulfur can make 2, 4 or 6 bonds. In fact, it is a multivalent atom, meaning it can make different number of bonds, up to 6, depending on the chemical formula.Symbol of Sulfur Archives Dynamic Periodic Table of Elements and
PPT Catalyst (5 minutes) PowerPoint Presentation, free download ID
Question 51110 Socratic
Ionic Bonding Elements are the simplest substances There
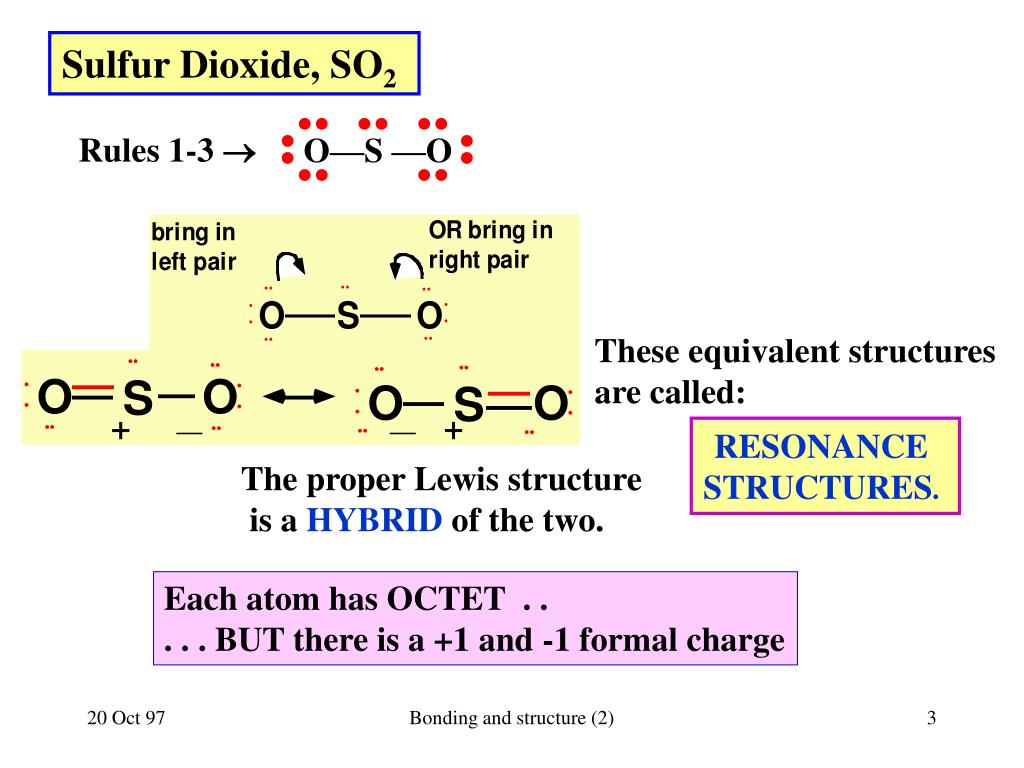
How to Find the Valence Electrons for SO2 (Sulfur Dioxide)?
PPT Chemical Bonding and Molecular Structure (Chapter 9) PowerPoint
Why can sulfur bond 6 times?
Molecules Free FullText Thiosulfoxide (Sulfane) Sulfur New
SF4 Molecular Geometry, Lewis Structure, Bond Angles and Polarity
How Many Covalent Bonds Can Sulfur Form CANZC
Related Post: