How Many Bonds Can Carbon Form With Other Elements
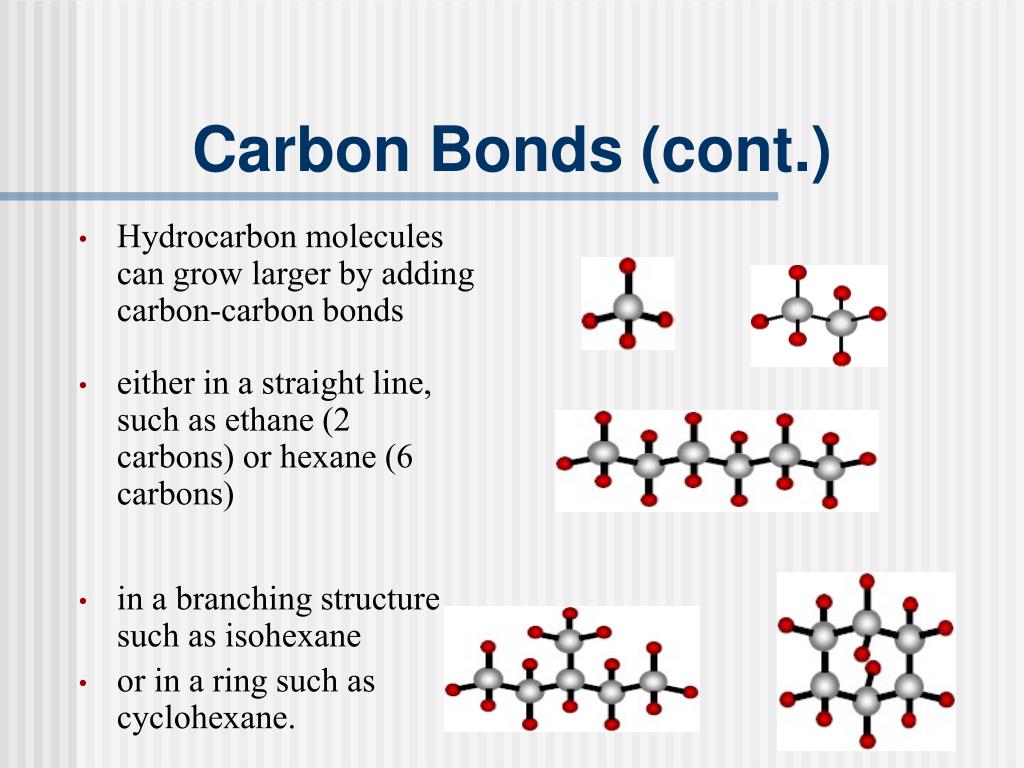
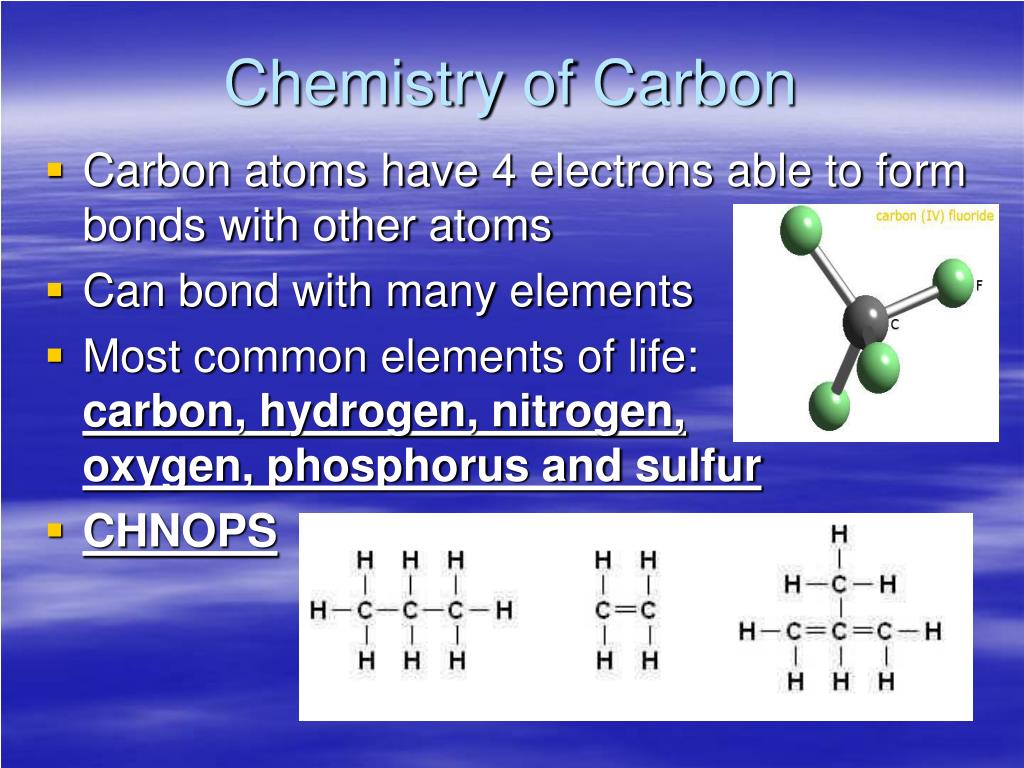
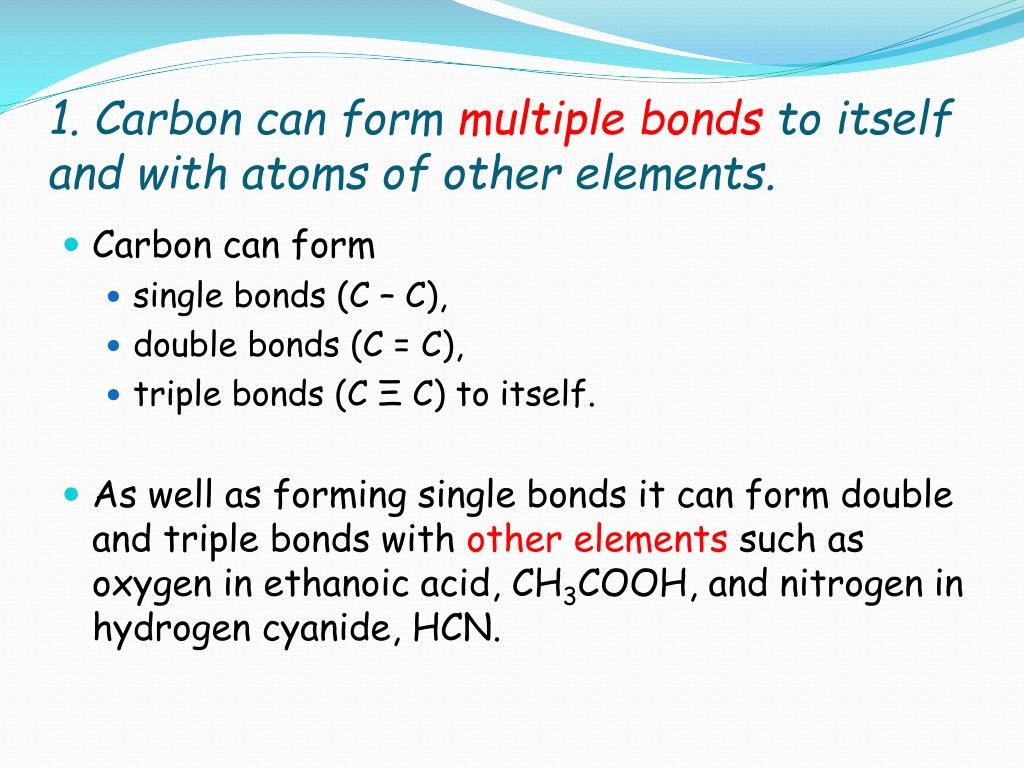
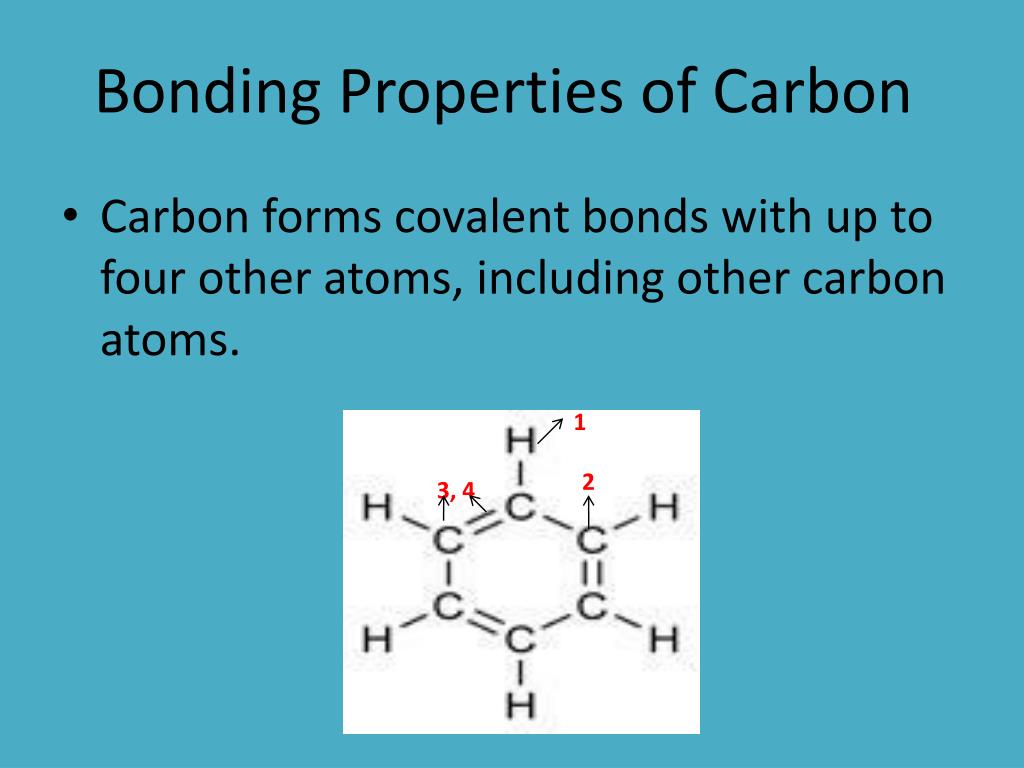
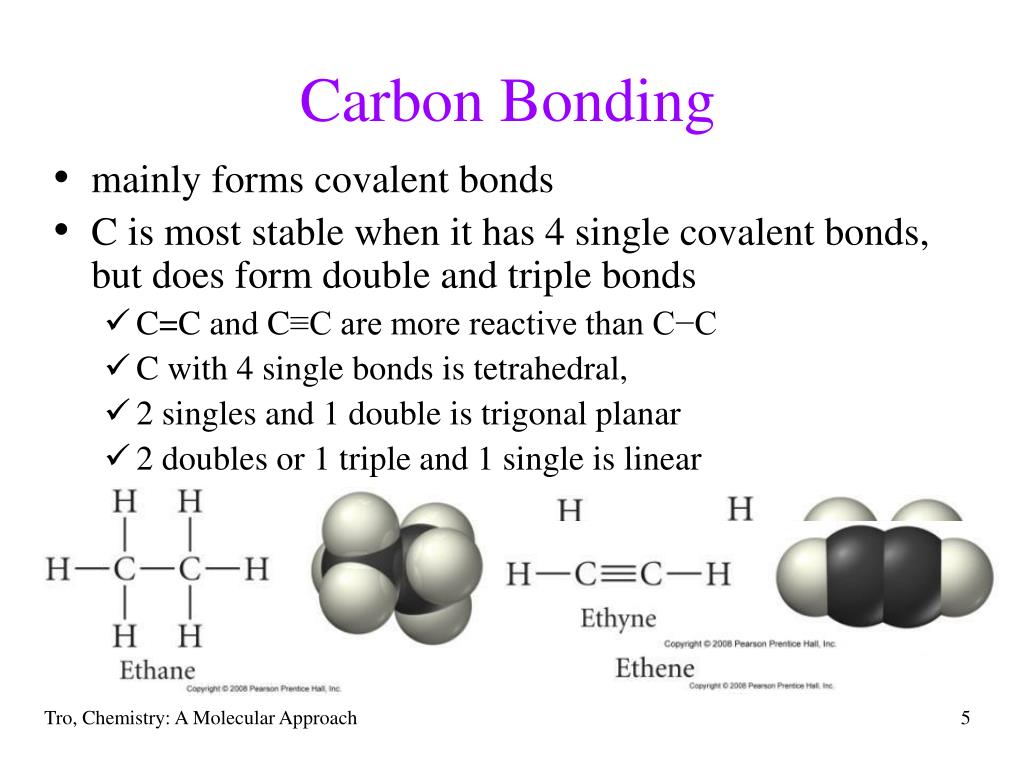
How Many Bonds Can Carbon Form With Other Elements - Web carbon can form single, double, or even triple bonds with other carbon atoms. Carbon has four valence electrons, so it can achieve a full outer energy level by. Web carbon forms covalent bonds with atoms of carbon or other elements. Therefore, carbon molecules share their 4 valence electrons. The simplest organic carbon molecule. Web in most stable compounds of carbon (and nearly all stable organic compounds), carbon obeys the octet rule and is tetravalent, meaning that a carbon atom forms a total of four. Therefore, it can form four covalent bonds with other atoms or molecules. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. It has four electrons in its outer shell which it can use for bonding, and needs to gain four in order to fill its outer shell. Web the four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living. Carbon forms covalent bonds with atoms of carbon or other elements. Therefore, it can form four covalent bonds with other atoms or molecules. There is a quick way to work out how many covalent bonds an element. Therefore, carbon molecules share their 4 valence electrons. Web atoms of different elements will form either one, two, three or four covalent bonds. Web carbon can form four covalent bonds to create an organic molecule. It has four electrons in its outer shell which it can use for bonding, and needs to gain four in order to fill its outer shell. Web carbon has four electrons in its outermost shell and can form four bonds. Carbon and hydrogen can form hydrocarbon chains or. In a double bond, they share two. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. There is a great diversity of carbon compounds, ranging in size from just one to thousands of atoms. Web carbon can form single, double, or even triple bonds with other carbon atoms. Web in most. There is a great diversity of carbon compounds, ranging in size from just one to thousands of atoms. With hydrogen, nitrogen, oxygen, and other heteroatoms. Therefore, it can form four covalent bonds with other atoms or molecules. Web carbon has four electrons in its outermost shell and can form four bonds. Carbon’s ability to form bonds with four other atoms. There is a quick way to work out how many covalent bonds an element. As with all covalent bonds,. The simplest carbon molecule is methane (ch 4 ), depicted here. There is a great diversity of carbon compounds, ranging in size from just one to thousands of atoms. Web carbon contains four electrons in its outer shell. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web the four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living. Carbon has four valence electrons, so it can achieve a full outer. There is a great diversity of carbon compounds, ranging in size from just one to thousands of atoms. Web carbon can form four covalent bonds to create an organic molecule. Web carbon has four electrons in its outermost shell and can form four bonds. Carbon and hydrogen can form hydrocarbon chains or rings. Therefore, carbon molecules share their 4 valence. Web carbon can form single, double, or even triple bonds with other carbon atoms. Carbon and hydrogen can form hydrocarbon chains or rings. The simplest carbon molecule is methane (ch 4 ), depicted here. The simplest organic carbon molecule. Web in most stable compounds of carbon (and nearly all stable organic compounds), carbon obeys the octet rule and is tetravalent,. Web no, carbon is not the only one that can bond to itself. Carbon and hydrogen can form hydrocarbon chains or rings. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. Web atoms of different elements will form either one, two, three or four. Therefore, it can form four covalent bonds with other atoms or molecules. Therefore, carbon molecules share their 4 valence electrons. Web 4 rows for example, each atom of a group 14 element has four electrons in its outermost shell and. Web well, carbon can form up to four covalent bonds. Web to form ionic bonds, carbon molecules must either gain. There is a quick way to work out how many covalent bonds an element. Web carbon forms covalent bonds with atoms of carbon or other elements. Therefore, it can form four covalent bonds with other atoms or molecules. Carbon and hydrogen can form hydrocarbon chains or rings. Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web 4 rows for example, each atom of a group 14 element has four electrons in its outermost shell and. Web carbon can form single, double, or even triple bonds with other carbon atoms. Carbon has four valence electrons, so it can achieve a full outer energy level by. It has four electrons in its outer shell which it can use for bonding, and needs to gain four in order to fill its outer shell. There is a great diversity of carbon compounds, ranging in size from just one to thousands of atoms. It's a unique property of some elements mainly the group 14 elements like silicon, germanium, arsenic etc. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Web no, carbon is not the only one that can bond to itself. Web the four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions, accounting for the importance of carbon in living. Therefore, carbon molecules share their 4 valence electrons. Functional groups are groups of atoms. Web carbon can form four covalent bonds to create an organic molecule. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. The simplest carbon molecule is methane (ch 4 ), depicted here.PPT Organic Chemistry Functional Groups PowerPoint Presentation
PPT Biochemistry PowerPoint Presentation, free download ID89333
PPT Carbon Compounds PowerPoint Presentation, free download ID2319022
PPT Biochemistry PowerPoint Presentation, free download ID89333
PPT Carbon Compounds PowerPoint Presentation, free download ID5444669
PPT ORGANIC PowerPoint Presentation, free download ID2170193
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
PPT 2.3 CarbonBased Molecules PowerPoint Presentation, free
PPT Chapter 20 Organic Chemistry PowerPoint Presentation, free
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
Related Post: