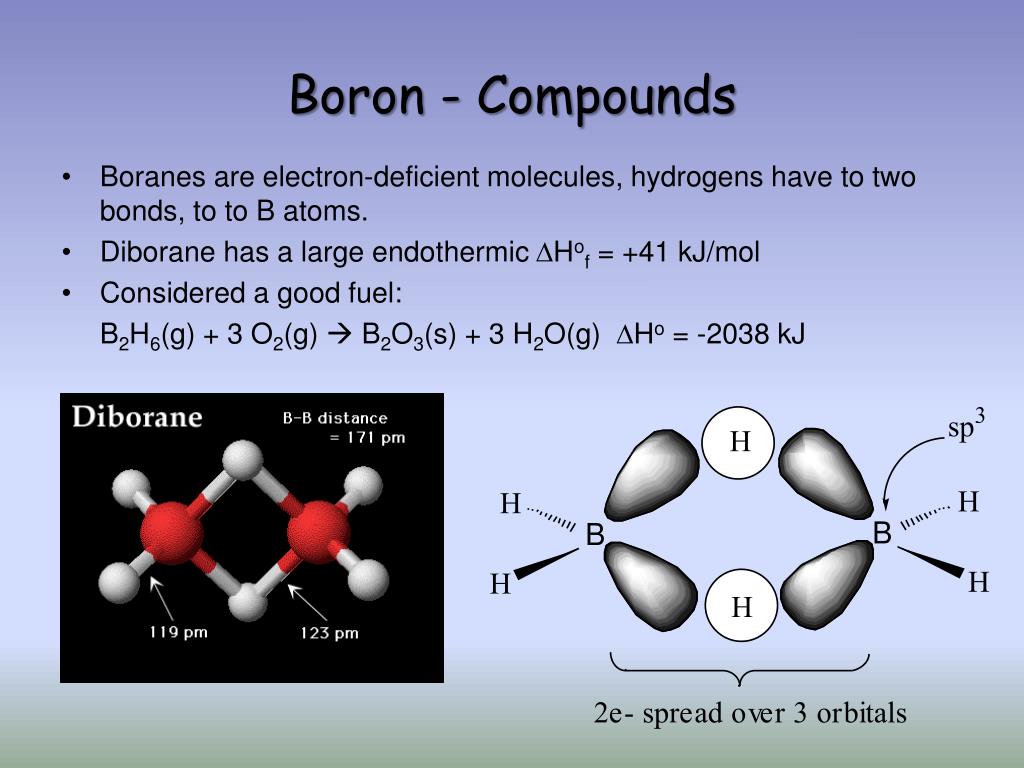
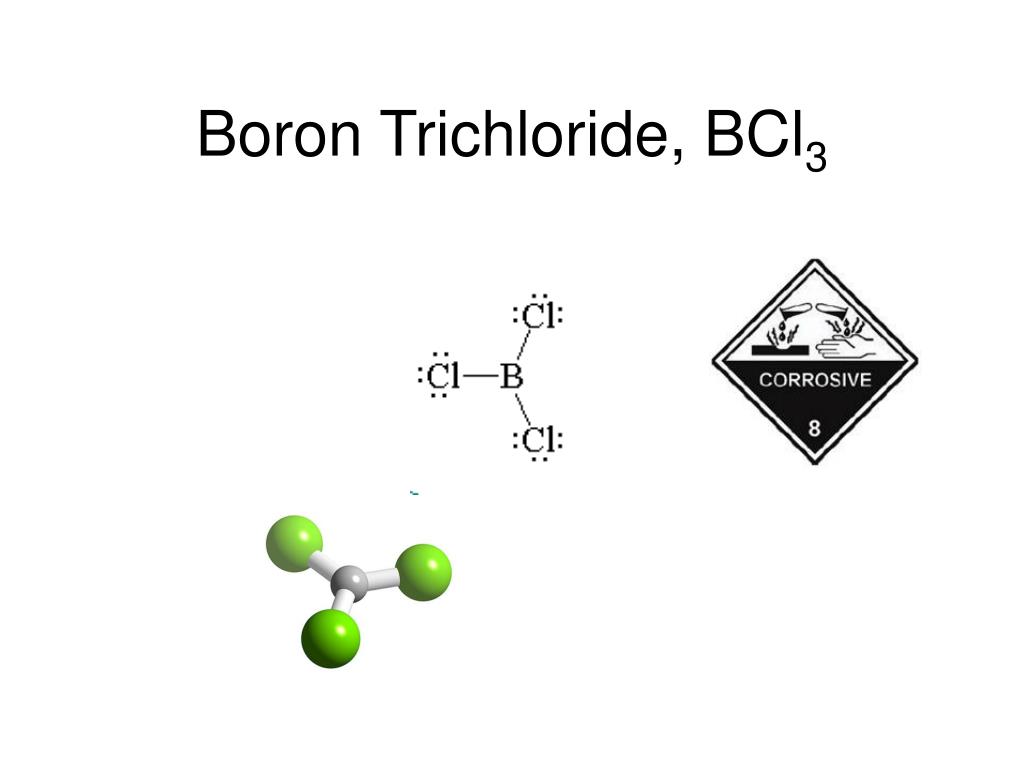
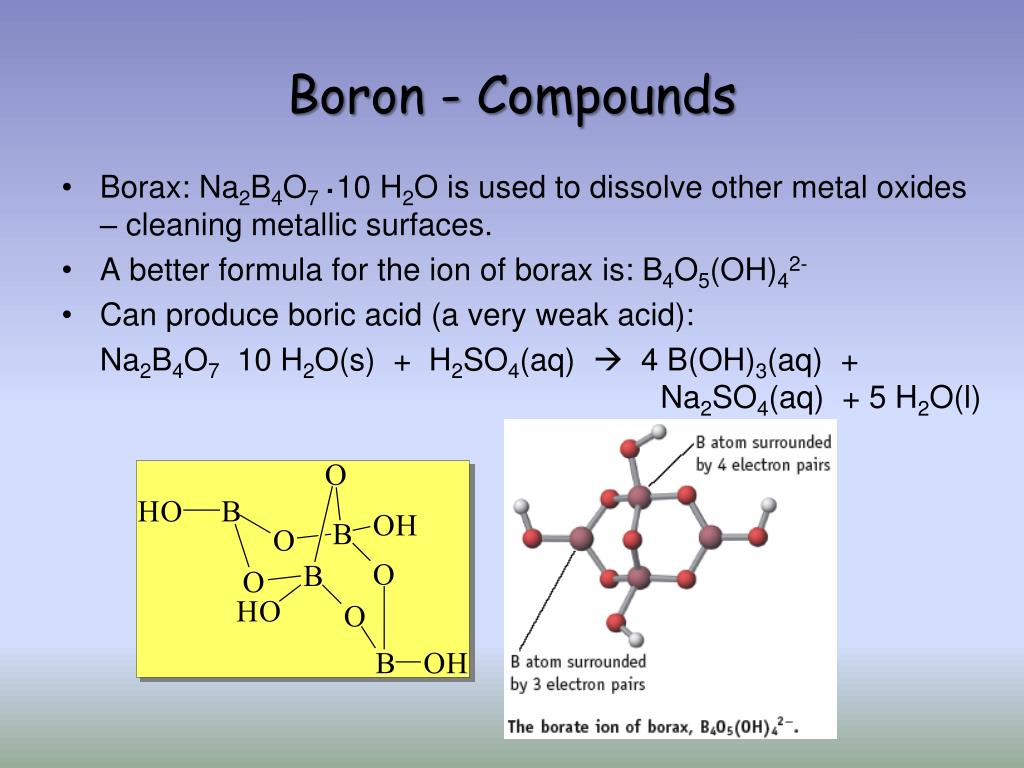
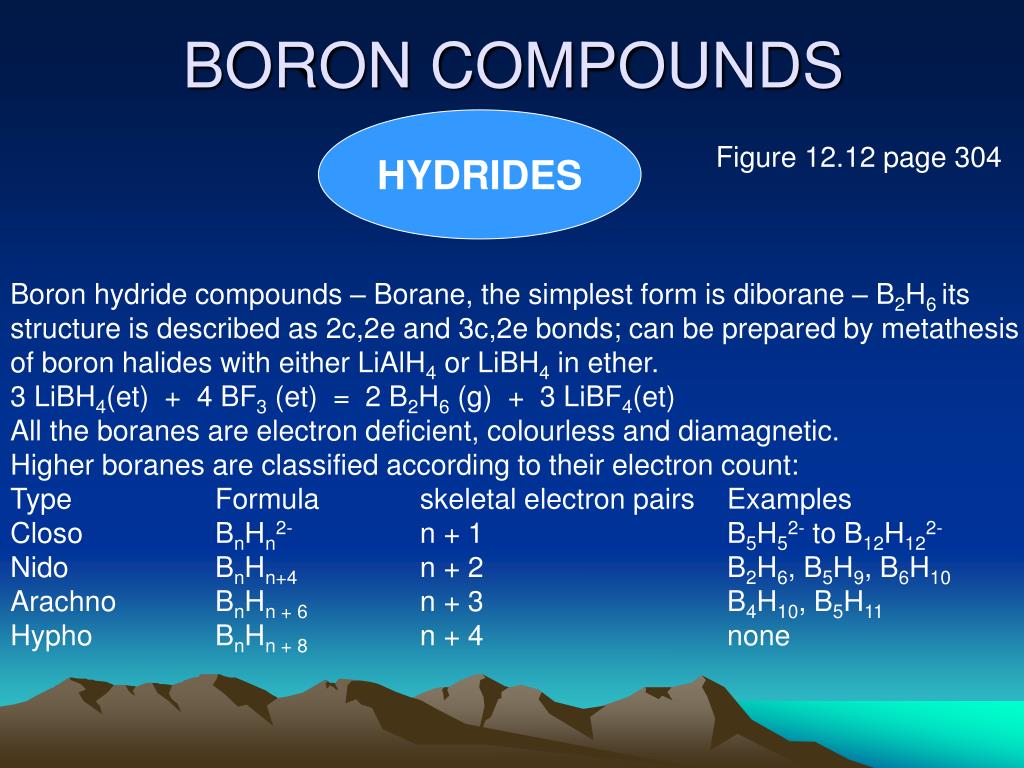
How Many Bonds Can Boron Form
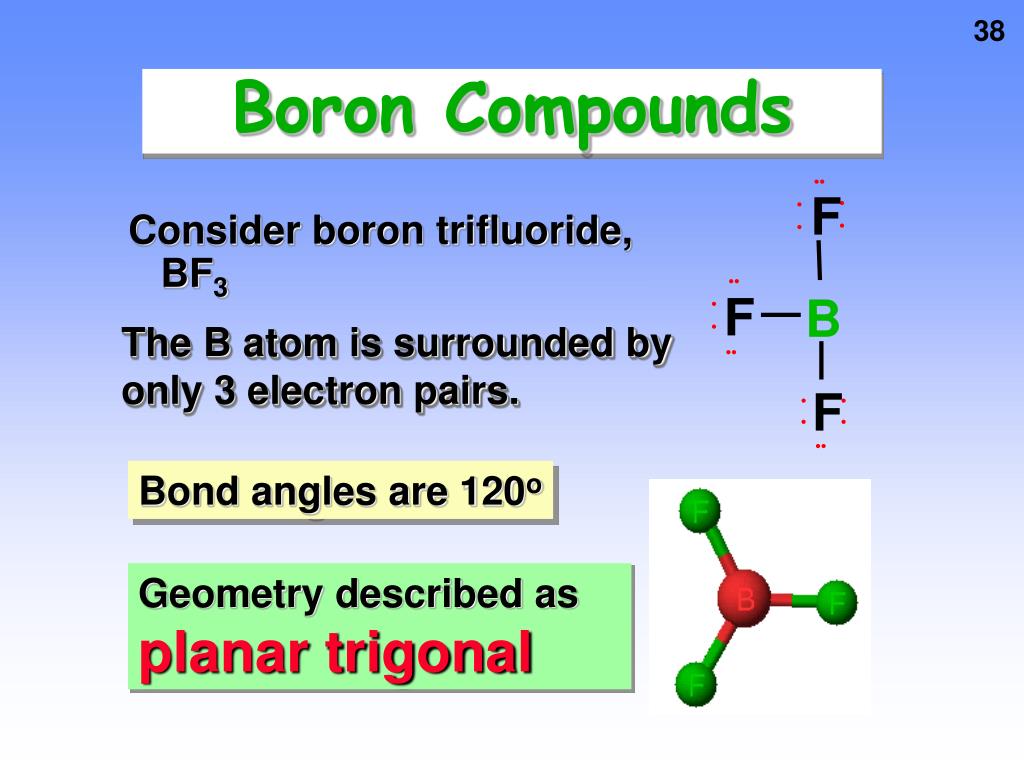
How Many Bonds Can Boron Form - Web how many single bonds can boron form? Group 4a elements form 4 bonds. Using the same explanation for the rest will help explain how elements form the given number of bonds: Boron (b) can typically form three bonds. These compounds do not occur in nature. Many of the boranes readily oxidise on. Web since it takes one electron from each atom to form a single bond, we can expect boron to form three bonds since it has three valence electrons. The first three ionization energies of boron, however, are much too high to allow. Web it follows, therefore, that an atom will tend to make as many covalent bonds as possible. For example, beryllium can form two covalent bonds, resulting in only four electrons in its. We know that boron is an element whose atomic number is 5. For example, beryllium can form two covalent bonds, resulting in only four electrons in its. Group 6a elements form 2 bonds. The boron centre has a formal negative charge. Web boron has a charge of 5. Web the most common examples are the covalent compounds of beryllium and boron. These compounds do not occur in nature. In the case of boron in bf 3, three bonds is the maximum possible. Two of them are core electrons and the remaining 3 are valence electrons. Web it follows, therefore, that an atom will tend to make as many. For example, elements such as boron or beryllium often form compounds in which the central atom is. Using the same explanation for the rest will help explain how elements form the given number of bonds: Web up to 4. This is balanced by 5 electrons. Web boron, chemical element that is a semimetal essential to plant growth and of wide. You might perhaps wonder why boron doesn't form ionic. Web boranes are chemical compounds of boron and hydrogen, with the generic formula of b x h y. Web boron is highly electronegative, and wants to form compounds with hydrogen atoms. These compounds do not occur in nature. It has 3 valence electrons in. Two of them are core electrons and the remaining 3 are valence electrons. Web in the case of boron in bf 3, three bonds is the maximum possible because boron only has 3 electrons to share. In the case of boron in bf 3, three bonds is the maximum possible. Web as a result, oxygen will make 2 bonds. Web. For example, elements such as boron or beryllium often form compounds in which the central atom is. Web how many single bonds can boron form? Adhesives, cement, disinfectants, fertilizers, etc. Web the most common examples are the covalent compounds of beryllium and boron. Web boranes are chemical compounds of boron and hydrogen, with the generic formula of b x h. Web while most atoms obey the duet and octet rules, there are some exceptions. Group 5a elements form 3 bonds. Boron has an atomic number of 5 and an electron configuration of 1s 2 2s 2 2p 1. The valence electrons may participate in. Boron trifluoride consists of a central boron atom with three single bonds to fluorine atoms(see figure. Adhesives, cement, disinfectants, fertilizers, etc. Web boron is highly electronegative, and wants to form compounds with hydrogen atoms. These compounds do not occur in nature. Boron (b) can typically form three bonds. Web as a result, oxygen will make 2 bonds. Web up to 4. The boron centre has a formal negative charge. Answer verified 300.3k + views hint: For example, beryllium can form two covalent bonds, resulting in only four electrons in its. Using the same explanation for the rest will help explain how elements form the given number of bonds: Web boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Group 5a elements form 3 bonds. Boron trifluoride consists of a central boron atom with three single bonds to fluorine atoms(see figure below). Web the most common examples are the covalent compounds of beryllium and boron. Web while most atoms obey the duet. Web boron has a charge of 5. Many of the boranes readily oxidise on. Group 6a elements form 2 bonds. Group 5a elements form 3 bonds. These compounds do not occur in nature. For example, beryllium can form two covalent bonds, resulting in only four electrons in its. The first three ionization energies of boron, however, are much too high to allow. In its compounds boron shows an oxidation state of +3. Two of them are core electrons and the remaining 3 are valence electrons. Web since it takes one electron from each atom to form a single bond, we can expect boron to form three bonds since it has three valence electrons. This is balanced by 5 electrons. Web as a result, oxygen will make 2 bonds. The boron centre has a formal negative charge. Web boranes are chemical compounds of boron and hydrogen, with the generic formula of b x h y. Web the most common examples are the covalent compounds of beryllium and boron. Web instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that contain multicenter bonds, including metal borides,. Web boron is highly electronegative, and wants to form compounds with hydrogen atoms. Web it follows, therefore, that an atom will tend to make as many covalent bonds as possible. Web boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron has an atomic number of 5 and an electron configuration of 1s 2 2s 2 2p 1.PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free
PPT Chemistry Bonding PowerPoint Presentation, free download ID
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free
Boron Properties, Uses, & Facts Britannica
Unprecedented formation of a boronboron covalent bond opens a new
SOME IMPORTANT COMPOUNDS OF BORON YouTube
PPT THE GROUP 13 ELEMENTS PowerPoint Presentation, free download ID
Boron Electron Configuration And Full Orbital Diagram
Coordinate Covalent Bond Dative Bond Boron Atom, Ionic Compound
PPT MOLECULAR GEOMETRY PowerPoint Presentation, free download ID
Related Post: