How Do Metals Form Ions
How Do Metals Form Ions - Web ions are formed as a result of this addition or removal of electrons from the atom. Ions form primarily through loss of \(s\) electrons. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. How do transition metals form ions? Web metal ions in aqueous solution. Once a protein and a solid surface form. Ions form when atoms lose or gain electrons to obtain a full outer shell: Web group 14 elements form none. Web how do transition metals form ions? Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Web it consists of $\mathrm{zn}^{2+}$ ions. Web metal atoms lose electrons to form positive ions (cations close cation an atom or group of atoms that have lost electrons and become positively charged. Once a protein and a solid surface form. Web group 14 elements form none. These ions are immersed in a sea of electrons, which balances their charge, holds. Web transition metals have unfilled inner \(d\) electron shells. Web metal atoms lose electrons to form positive ions (cations close cation an atom or group of atoms that have lost electrons and become positively charged. Web how do transition metals form ions? Metal atoms lose electrons to form. Ions form primarily through loss of \(s\) electrons. The number lost is the same as. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Ions form primarily through loss of \(s\) electrons. Web transition metals have unfilled inner \(d\) electron shells. Web transitional metal ions, such as cu 2+,. Web metals form positive ions (cations). Web transitional metal ions, such as cu 2+, zn 2+, ni 2+, and co 2+, can form coordination bonds with the imidazolyl group of histidine. The transition metals are an interesting and challenging group of elements. These ions are immersed in a sea of electrons, which balances their charge, holds them together, and also. They have perplexing patterns of electron distribution. Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; The transition metals are an interesting and challenging. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so. Web metal atoms lose electrons to form positive ions (cations close cation an atom or group of atoms that have lost electrons and become positively charged. Group 18 elements form none. The number lost is the same as. Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. When. Web transition metals have unfilled inner \(d\) electron shells. Web atomic structure and bonding related to properties of materials. Web metal ions in aqueous solution. Web metal atoms lose electrons to form positive ions (cations close cation an atom or group of atoms that have lost electrons and become positively charged. Web the name of a metal ion is the. Ions are formed when atoms lose or gain electrons to obtain the stable electron arrangement of a. By combination of ions with other particles; These ions are immersed in a sea of electrons, which balances their charge, holds them together, and also is responsible for such. Metal atoms lose electrons to form. The transition metals are an interesting and challenging. Web group 14 elements form none. A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. Web metal ions in aqueous solution. Web ion is an atom or group of atoms with a positive or negative charge. Ions are formed when atoms lose or gain electrons. Web transition metals have unfilled inner \(d\) electron shells. Ions form when atoms lose or gain electrons to obtain a full outer shell: By combination of ions with other particles; Web atomic structure and bonding related to properties of materials. Web ion is an atom or group of atoms with a positive or negative charge. How do transition metals form ions? Web ions are formed as a result of this addition or removal of electrons from the atom. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas,. These ions are immersed in a sea of electrons, which balances their charge, holds them together, and also is responsible for such. When atoms of nonmetal elements form ions, they. Group 18 elements form none. Web ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; Ions form when atoms lose or gain electrons to obtain a full outer shell: Metal atoms lose electrons to form. Web ion is an atom or group of atoms with a positive or negative charge. Web metal ions in aqueous solution. Thus metals are electropositive elements with relatively low ionization energies. Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. Ions form primarily through loss of \(s\) electrons. Once a protein and a solid surface form. Web it consists of $\mathrm{zn}^{2+}$ ions. Ions are formed when atoms lose or gain electrons to obtain the stable electron arrangement of a. Ions are formed when atoms lose or gain negatively charged electrons.Ionic Bond Definition, Types, Properties & Examples
Ions
Periodic Table Ions List Periodic Table Timeline
Ionic bonding Wikipedia
Chem Ions Scientific Tutor
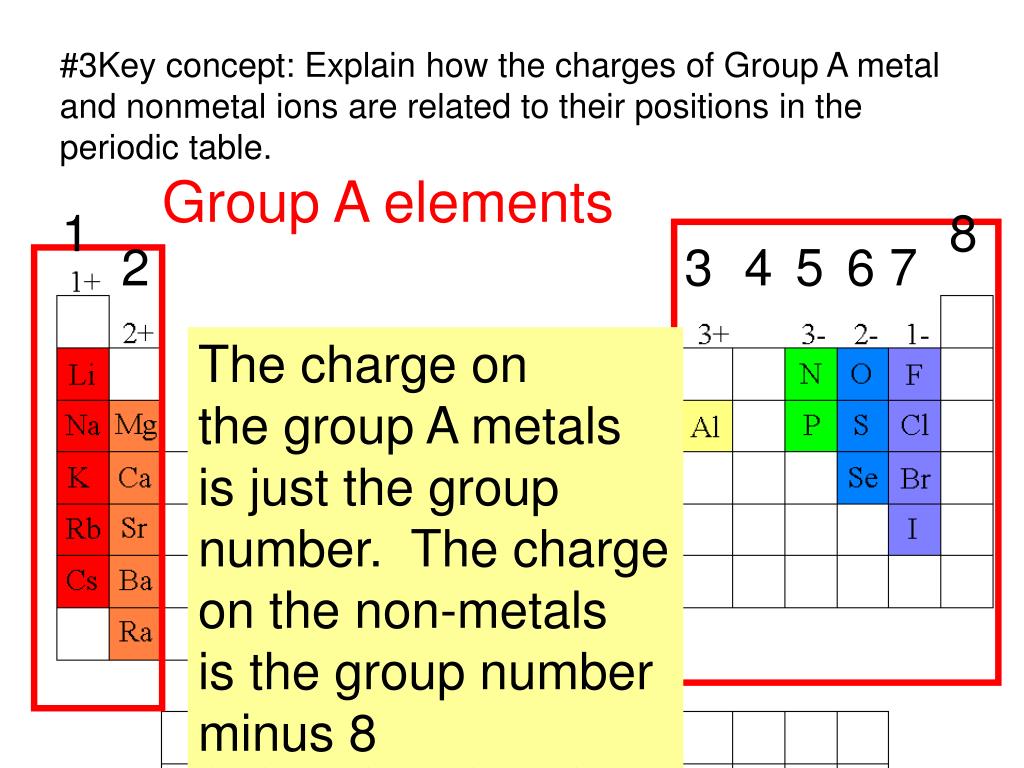
PPT 1 Name the ions formed by these elements and classify them as
Naming Simple Ionic Compounds Pathways to Chemistry
Metallic Bond — Formation & Compounds Expii
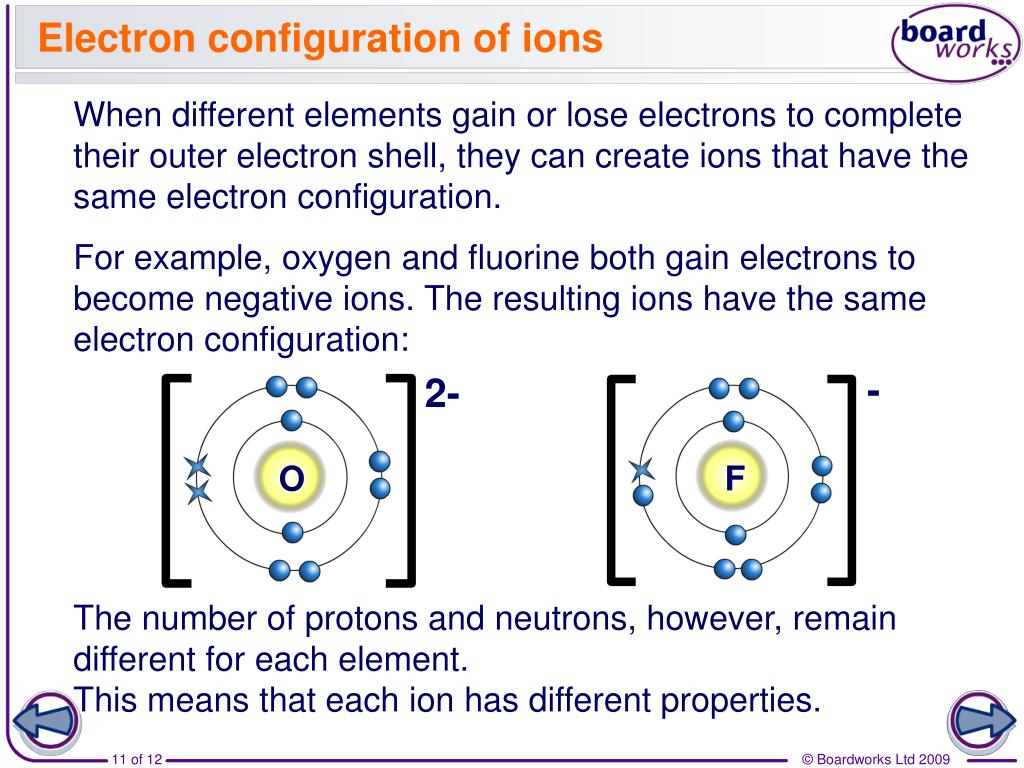
PPT How do atoms form ions? PowerPoint Presentation, free download
Ionic Bonding Presentation Chemistry
Related Post:







.PNG)