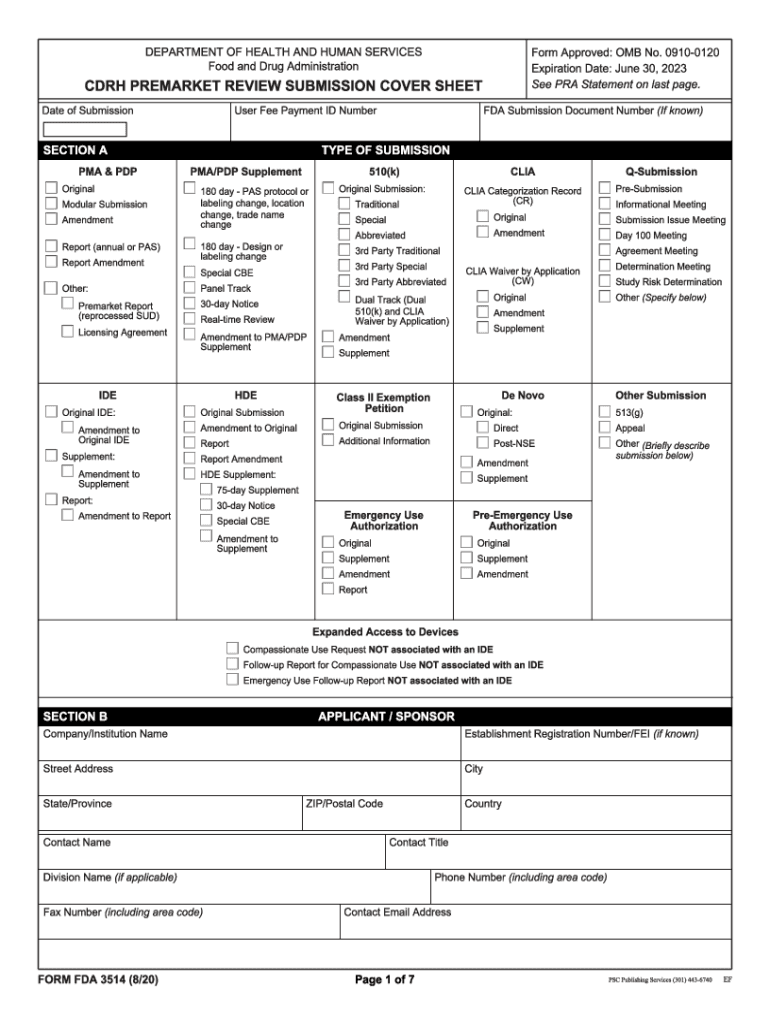
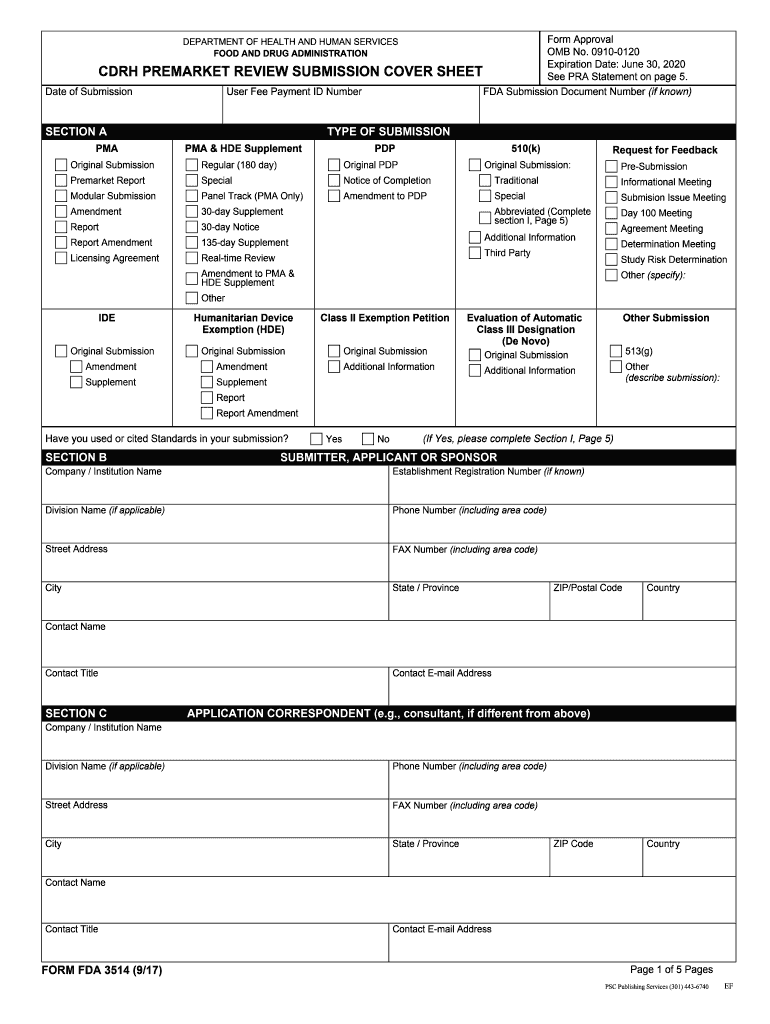
Form Fda 3514
Form Fda 3514 - Web form fda 3514, or the cdrh premarket review submission cover sheet, is a voluntary form used to help provide basic administrative info for all types of premarket. Web quick steps to complete and design form 3514 fda online: Web what is fda form 3514? The form provides the fda with the information required of applicants who submit certain human drug, biological product, and device. Form fda 3514 (1/13) author: Start completing the fillable fields and. Web up to $3 cash back fda form 3514 was developed to assist respondents in organizing 510k data for submission to. Web section c of cdrh premarket review submission cover sheet (form fda 3514) or in section b (if section c is blank on the cover sheet form), or in the cover. Web applicants should clearly indicate their use of standards in premarket submissions by appropriately identifying any referenced standards in their cdrh. Web medical device user fee cover sheet (form fda 3601) (2) center for devices and radiological health (cdrh) premarket review submission cover sheet. Web • cdrh premarket review submission cover sheet (form fda 3514) • table of contents • detailed device description • proposed intended use/indications for use It helps to identify the type, content, and purpose of the submission, as well as. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing 510(k) information for submission to fda. Web. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. The form provides the fda with the information required of applicants who submit certain. Save or instantly send your ready documents. Ad download or email form fda 3514 & more fillable forms, register and subscribe now! Web what is fda form 3514? It helps to identify the type, content, and purpose of the submission, as well as. Easily fill out pdf blank, edit, and sign them. Web what is fda form 3514? Web section c of cdrh premarket review submission cover sheet (form fda 3514) or in section b (if section c is blank on the cover sheet form), or in the cover. It helps to identify the type, content, and purpose of the submission, as well as. Web up to $3 cash back fda form. Easily fill out pdf blank, edit, and sign them. Economic growth and income inequality kuznets pdf. Web form fda 3514, or the cdrh premarket review submission cover sheet, is a voluntary form used to help provide basic administrative info for all types of premarket. Web what is fda form 3514? Web without further ado, let’s jump into the first group. Cdrh premarket review submission cover sheet\r\n\(v5.5\) created date:. Web form fda 3514, or the cdrh premarket review submission cover sheet, is a voluntary form used to help provide basic administrative info for all types of premarket. Web applicants should clearly indicate their use of standards in premarket submissions by appropriately identifying any referenced standards in their cdrh. Start completing the. Web up to $3 cash back fda form 3514 was developed to assist respondents in organizing 510k data for submission to. The form provides the fda with the information required of applicants who submit certain human drug, biological product, and device. Web what is fda form 3514? Web • we revised and reformatted form fda 3514, ‘‘cdrh premarket review submission. Web form fda 3514 is a voluntary coversheet for premarket submissions of medical devices to the fda. Web applicants should clearly indicate their use of standards in premarket submissions by appropriately identifying any referenced standards in their cdrh. Web medical device user fee cover sheet (form fda 3601) (2) center for devices and radiological health (cdrh) premarket review submission cover. Web medical device user fee cover sheet (form fda 3601) (2) center for devices and radiological health (cdrh) premarket review submission cover sheet. Web without further ado, let’s jump into the first group. Web the completion of this premarket submission coversheet (form fda 3514) is voluntary and will not affect any food and drug administration (fda) decision concerning your. Cdrh. Web section c of cdrh premarket review submission cover sheet (form fda 3514) or in section b (if section c is blank on the cover sheet form), or in the cover. Save or instantly send your ready documents. The form provides the fda with the information required of applicants who submit certain human drug, biological product, and device. Web the. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing 510(k) information for submission to fda. Use the following instructions to download the form if. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. Form fda 3514 (1/13) author: Web section c of cdrh premarket review submission cover sheet (form fda 3514) or in section b (if section c is blank on the cover sheet form), or in the cover. Web medical device user fee cover sheet (form fda 3601) (2) center for devices and radiological health (cdrh) premarket review submission cover sheet. Web up to $40 cash back get the free fda form 3514 2017. Web form fda 3514, or the cdrh premarket review submission cover sheet, is a voluntary form used to help provide basic administrative info for all types of premarket. Easily fill out pdf blank, edit, and sign them. Web center for devices and radiological health (cdrh) premarket review submission cover sheet (form fda 3514) 510(k) cover letter to continue reading this ebook including a. Web applicants should clearly indicate their use of standards in premarket submissions by appropriately identifying any referenced standards in their cdrh. The form provides the fda with the information required of applicants who submit certain human drug, biological product, and device. Economic growth and income inequality kuznets pdf. Web • we revised and reformatted form fda 3514, ‘‘cdrh premarket review submission cover sheet,’’ to improve usability and to be inclusive of most medical. Cdrh premarket review submission cover sheet\r\n\(v5.5\) created date:. Use get form or simply click on the template preview to open it in the editor. An agency may not conduct or sponsor, and a person is not required to respond to, a. Ad download or email form fda 3514 & more fillable forms, register and subscribe now! It helps to identify the type, content, and purpose of the submission, as well as. Start completing the fillable fields and.510(k) PreMarket Notification Project
FDA Applications 12 Free Templates in PDF, Word, Excel Download
Free Special Power Of Attorney Pdf
Fda Form 3514 Fill Out and Sign Printable PDF Template signNow
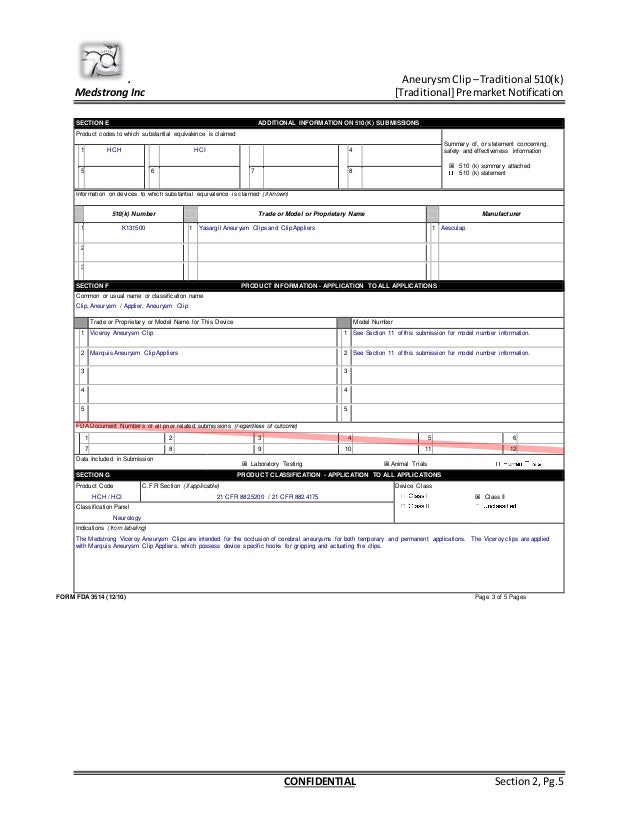
FDA 510(k) submission redacted
Fda Form 3514 Fill Out and Sign Printable PDF Template signNow
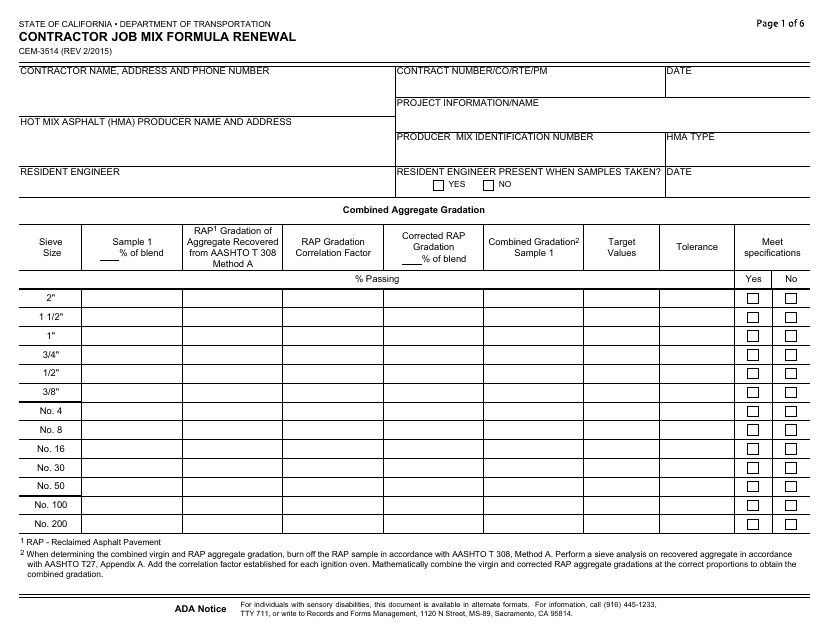
Form CEM3514 Download Fillable PDF or Fill Online Contractor Job Mix
FDA 510(k) submission redacted
PCrompton_510(k)
36 Fda Forms And Templates free to download in PDF
Related Post: