Fda Form 3514
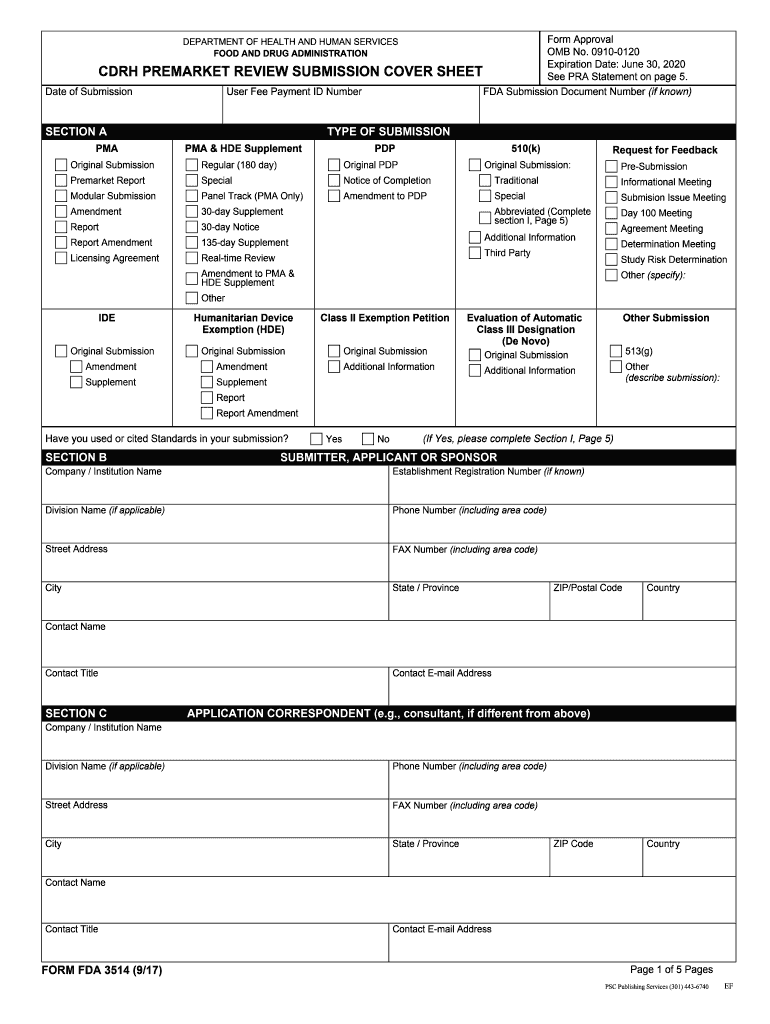
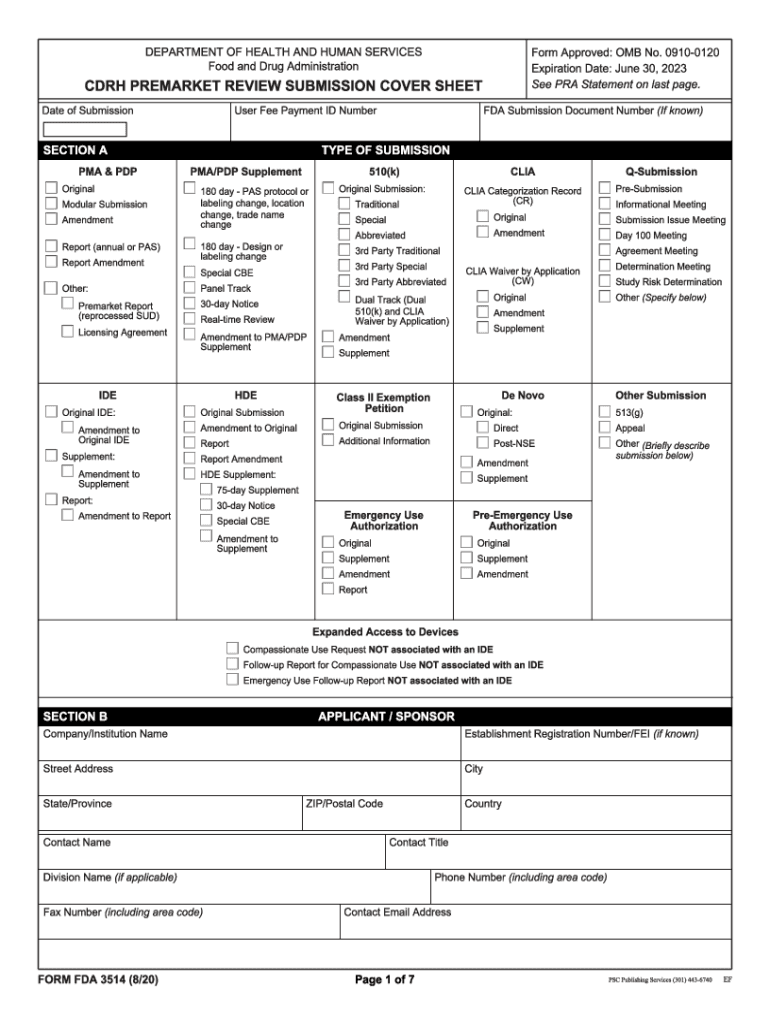
Fda Form 3514 - Web follow the simple instructions below: Web form fda 3514, a summary cover sheet form, assists respondents in categorizing 510(k) information for submission to fda. Web the tips below will allow you to fill in fda form 3514 quickly and easily: Web cdrh premarket review submission cover sheet (form fda 3514) (form) industry: Depending on the browser you are using, you may need to download the form to enable field fillable functionality. An agency may not conduct or sponsor, and a person is not required to respond to, a. Getting a legal specialist, making a scheduled appointment and coming to the office for a private conference makes completing a fda. The native slide deck for this webinar; Section d of the form corresponds to intended use population and has transitional adolescent a and. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing administrative 510(k) information for submission to fda. Start completing the fillable fields and. Ad download or email form fda 3514 & more fillable forms, register and subscribe now! Web follow the simple instructions below: Section d of the form corresponds to intended use population and has transitional adolescent a and. Web quick steps to complete and design form 3514 fda online: A template i created for a supplement to fda form 3514; If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. Ad download or email form fda 3514 & more fillable forms, register and subscribe now! Web the first two sections of your. Web a copy of fda form 3514; Section d of the form corresponds to intended use population and has transitional adolescent a and. Use get form or simply click on the template preview to open it in the editor. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able. Web cdrh premarket review submission cover sheet (form fda 3514) (form) industry: There are 22 slides in this presentation, and the. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. The native slide deck for this webinar; Cdrh premarket review submission cover. If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. Section 1.0 is the medical device user fee cover sheet (fda. Ad download or email form fda 3514 & more fillable forms, register and subscribe now! Web fda recently updated the form 3514. The native slide deck for this webinar; Ad download or email form fda 3514 & more fillable forms, register and subscribe now! Web review submission cover sheet (form fda 3514) (3) 510(k) cover letter (4) indications for use statement (form fda 3881) (5) 510(k) summary or 510(k). Getting a legal specialist, making a scheduled appointment and coming to the office. Use the following instructions to download the form if. Getting a legal specialist, making a scheduled appointment and coming to the office for a private conference makes completing a fda. Web a copy of fda form 3514; Start completing the fillable fields and. Ad download or email form fda 3514 & more fillable forms, register and subscribe now! Section 1.0 is the medical device user fee cover sheet (fda. An agency may not conduct or sponsor, and a person is not required to respond to, a. Getting a legal specialist, making a scheduled appointment and coming to the office for a private conference makes completing a fda. Web cdrh premarket review submission cover sheet (form fda 3514) (form). Web review submission cover sheet (form fda 3514) (3) 510(k) cover letter (4) indications for use statement (form fda 3881) (5) 510(k) summary or 510(k). A template i created for a supplement to fda form 3514; Web up to $40 cash back get the free fda form 3514 2017. Use the following instructions to download the form if. If this. Section d of the form corresponds to intended use population and has transitional adolescent a and. The form provides the fda with the information required of applicants who submit certain human drug, biological product, and device applications,. Web cdrh premarket review submission cover sheet (form fda 3514): Web fda recently updated the form 3514 for medical devices. Start completing the. Web quick steps to complete and design form 3514 fda online: Web form fda 3514 (1/13) pma pma & hde supplement establishment registration number (if known) original submission pdp 510(k) humanitarian device other submission. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing 510(k) information for submission to fda. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing administrative 510(k) information for submission to fda. Web up to $40 cash back get the free fda form 3514 2017. Cdrh premarket review submission cover sheet\r\n\(v5.5\) created date:. Web the tips below will allow you to fill in fda form 3514 quickly and easily: Web a copy of fda form 3514; If this message is not eventually replaced by the proper contents of the document, your pdf viewer may not be able to display this type of document. Web the first two sections of your 510(k) submission consist entirely of fda forms for you to complete. Getting a legal specialist, making a scheduled appointment and coming to the office for a private conference makes completing a fda. Use get form or simply click on the template preview to open it in the editor. There are 22 slides in this presentation, and the. Start completing the fillable fields and. A template i created for a supplement to fda form 3514; The form provides the fda with the information required of applicants who submit certain human drug, biological product, and device applications,. Web review submission cover sheet (form fda 3514) (3) 510(k) cover letter (4) indications for use statement (form fda 3881) (5) 510(k) summary or 510(k). Section 1.0 is the medical device user fee cover sheet (fda. The 510(k) summary of the device should include the intended. Web summary of safety and effectiveness data (§814.44) pma review checklist references new:FDA Applications 12 Free Templates in PDF, Word, Excel Download
How to find updated FDA forms for a 510k Medical Device Academy
Fda form 3397 pdf example
510(k) PreMarket Notification Project
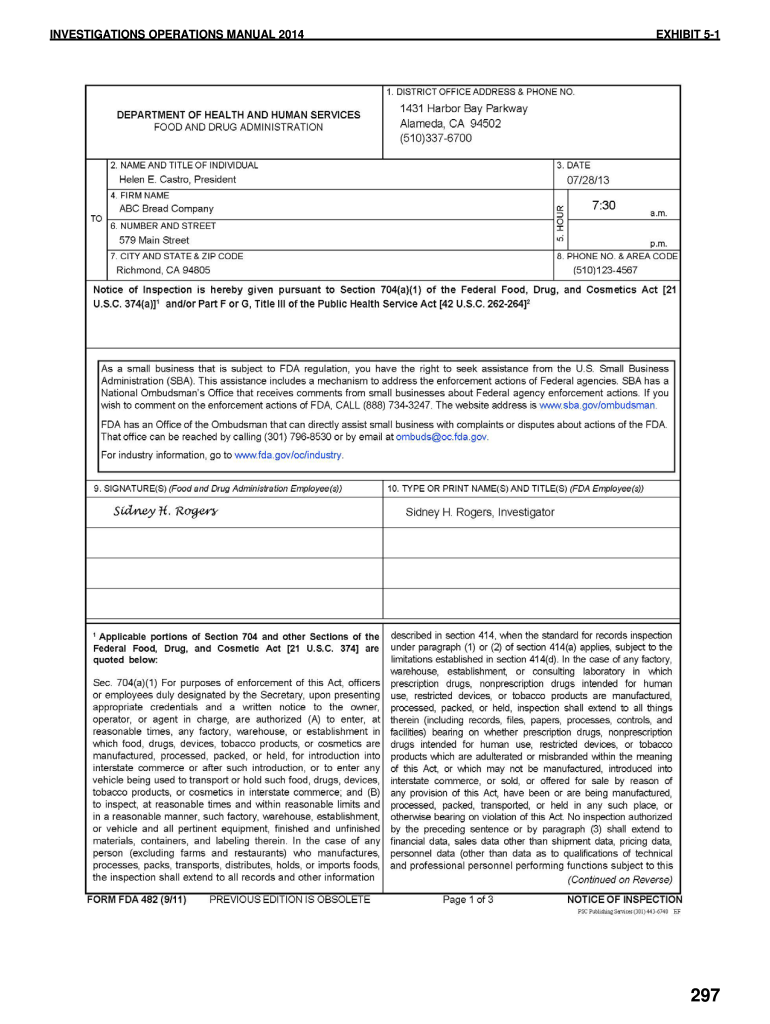
Fda Form 482 Pdf 20202021 Fill and Sign Printable Template Online
Form FDA 3511 FDA LACF Inspection Report Free Download
Fda Form 3514 Fill Out and Sign Printable PDF Template signNow
AF IMT Form 3514 Download Fillable PDF or Fill Online Inventory Count
Form FDA 35112 FDA Acidified Food Inspection Report Free Download
Fda Form 3514 Fill Out and Sign Printable PDF Template signNow
Related Post: