Fda Form 3455
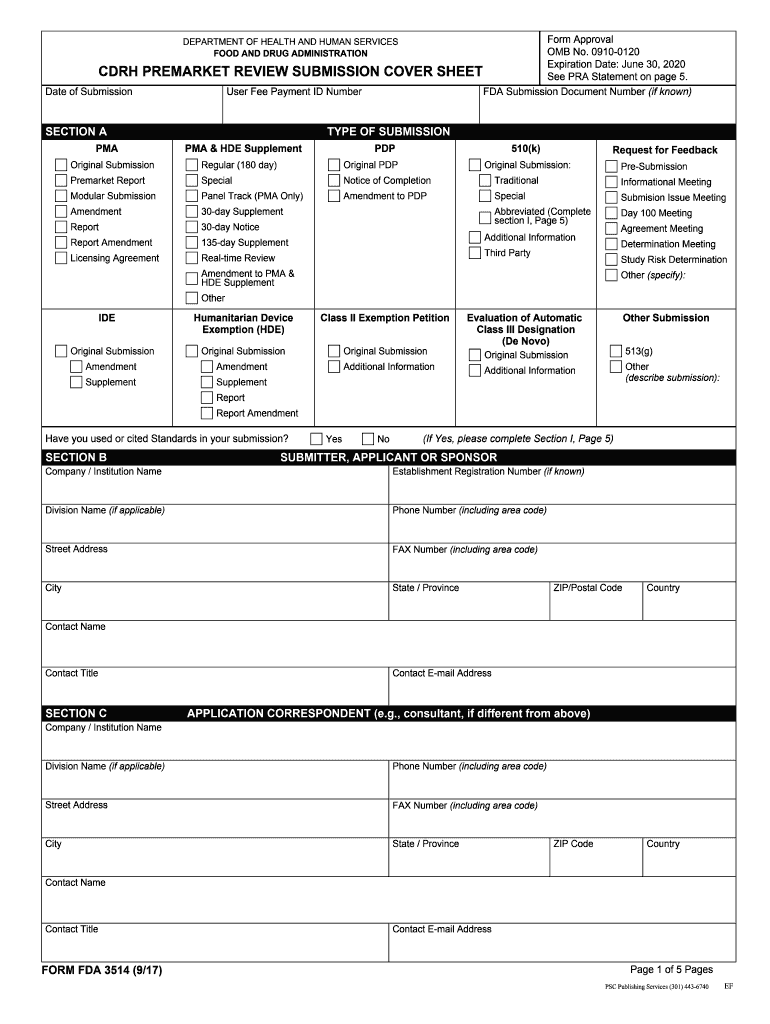
Fda Form 3455 - Web complete fda form 3455 online with us legal forms. Web when certification is not possible and disclosure is made using the following form fda 3455, the applicant must describe the financial arrangements or interests and the steps. The completed form must identify both the. Save or instantly send your ready documents. For any clinical investigator defined in § 54.2(d) for whom the applicant does not submit the certification described in paragraph (a)(1) of this section,. Complete form 3454 if none of the investigators have any fda required disclosures. When a reportable financial arrangement exists, the sponsor must complete form fda 3455 (figure 2). Web this form provides the fda with important information about the clinical trial, such as the protocol, investigator qualifications, and other important information. Web form fda 3455: Easily fill out pdf blank, edit, and sign them. For any clinical investigator defined in § 54.2(d) for whom the applicant does not submit the certification described in paragraph (a)(1) of this section,. Financial interest and arrangements of clinical investigators) form. Financial interest and arrangements of clinical investigator) form 3455 (disclosure: Web this form provides the fda with important information about the clinical trial, such as the protocol, investigator. Web form fda 3455 (rev. Financial interests and arrangements of clinical investigators fda form 3455 format for ide progress reports (06/01/1996) goals and. Ind checklist for ind submission. Easily fill out pdf blank, edit, and sign them. Save or instantly send your ready documents. Ind checklist for ind submission. Financial interest and arrangements of clinical investigators) form. Complete form 3455 if any clinical investigator has a financial disclosure. (a) the food and drug administration (fda) evaluates clinical studies submitted in marketing applications, required by law, for new human. Web when certification is not possible and disclosure is made using the following form fda 3455,. Financial interests and arrangements of clinical investigators fda form 3455 format for ide progress reports (06/01/1996) goals and. Getting a authorized professional, making a scheduled appointment and coming to the business office for a personal conference makes doing a. Web protecting study volunteers in clinical research by cynthia mcguire dunn; The completed form must identify both the. Web this form. Financial interest and arrangements of clinical investigator) form 3455 (disclosure: Web protecting study volunteers in clinical research by cynthia mcguire dunn; Financial interest and arrangements of clinical investigators fda 3674 (pdf 2.6mb) certification of compliance under 42. Web when certification is not possible and disclosure is made using the following form fda 3455, the applicant must describe the financial arrangements. Getting a authorized professional, making a scheduled appointment and coming to the business office for a personal conference makes doing a. Use the following instructions to download the form if. The completed form must identify both the. Web follow the simple instructions below: Web protecting study volunteers in clinical research by cynthia mcguire dunn; Financial interest and arrangements of clinical investigators fda 3674 (pdf 2.6mb) certification of compliance under 42. Web protecting study volunteers in clinical research by cynthia mcguire dunn; When a reportable financial arrangement exists, the sponsor must complete form fda 3455 (figure 2). Web follow the simple instructions below: Web form fda 3455 (rev. Financial interest and arrangements of clinical investigators fda 3674 (pdf 2.6mb) certification of compliance under 42. Web this form provides the fda with important information about the clinical trial, such as the protocol, investigator qualifications, and other important information. Financial interest and arrangements of clinical investigator) form 3455 (disclosure: Use the following instructions to download the form if. Ind checklist. Web complete fda form 3454 or 3455 ; Financial interests and arrangements of clinical investigators form fda 3674: Web follow the simple instructions below: Complete form 3455 if any clinical investigator has a financial disclosure. Web completely and accurately disclose, using form fda 3455, the nature of those interests and arrangements to the agency and describe any steps taken to. Web form fda 3455 (rev. Certification of compliance submission of an ind. (a) the food and drug administration (fda) evaluates clinical studies submitted in marketing applications, required by law, for new human. Complete form 3454 if none of the investigators have any fda required disclosures. Save or instantly send your ready documents. Update this information during the study and for one year after study completion;. Web form fda 3455: (a) the food and drug administration (fda) evaluates clinical studies submitted in marketing applications, required by law, for new human. The completed form must identify both the. Save or instantly send your ready documents. Web form fda 3455. Ind checklist for ind submission. Complete form 3454 if none of the investigators have any fda required disclosures. Web follow the simple instructions below: Web this form provides the fda with important information about the clinical trial, such as the protocol, investigator qualifications, and other important information. When a reportable financial arrangement exists, the sponsor must complete form fda 3455 (figure 2). Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web form fda 3455 (rev. Financial interest and arrangements of clinical investigators fda 3674 (pdf 2.6mb) certification of compliance under 42. Financial interests and arrangements of clinical investigators form fda 3674: Web complete fda form 3454 or 3455 ; Complete form 3455 if any clinical investigator has a financial disclosure. Web completely and accurately disclose, using form fda 3455, the nature of those interests and arrangements to the agency and describe any steps taken to minimize the potential. Web when certification is not possible and disclosure is made using the following form fda 3455, the applicant must describe the financial arrangements or interests and the steps. For any clinical investigator defined in § 54.2(d) for whom the applicant does not submit the certification described in paragraph (a)(1) of this section,.Fda Form 3514 Fill Out and Sign Printable PDF Template signNow
Fda 1572 Template
Form 1571 Fill Out and Sign Printable PDF Template signNow
Form FDA 3455 Disclosure Financial Interest and Arrangements of
Form FDA 3511 FDA LACF Inspection Report Free Download
Form Fda 3454 Certification Financial Interests And Arrangements Of
Medwatch Form Fill Out and Sign Printable PDF Template signNow
Investigator Database Form Fill Online, Printable, Fillable, Blank
510(k) PreMarket Notification Project
Form FDA 3455 Disclosure Financial Interest and Arrangements of
Related Post: