Explain Why Liquids In A Test Tube Form A Meniscus
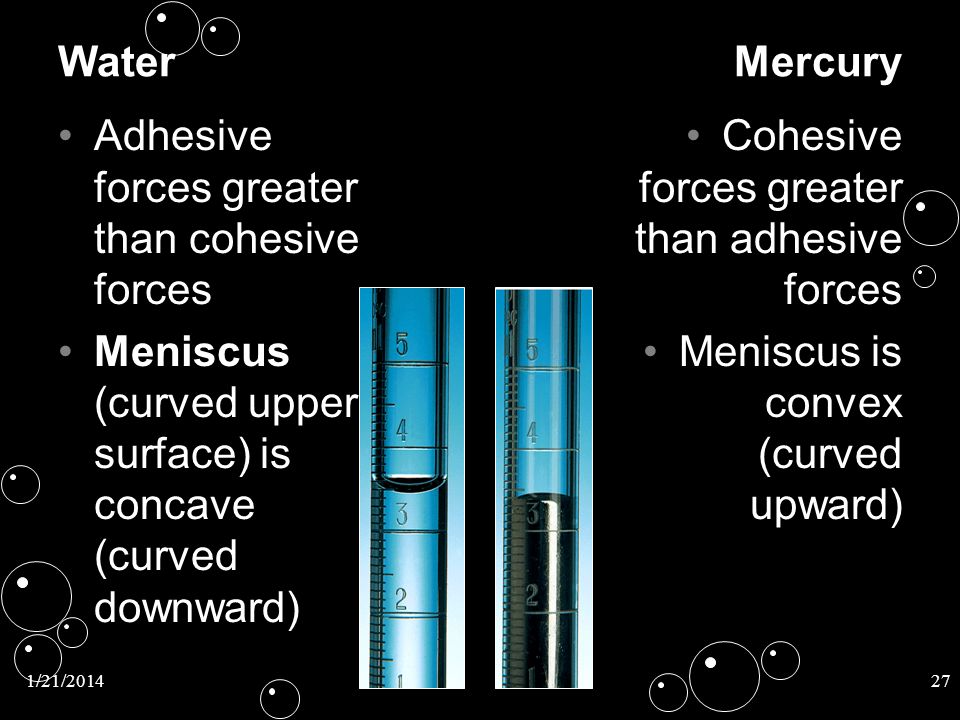
Explain Why Liquids In A Test Tube Form A Meniscus - The key to getting an accurate reading is to measure the center of the meniscus whether it. Surface tension and adhesive forces cause the formation of a meniscus. Most liquids have an attraction between their molecules that is stronger than the. Most liquids, including water, present a concave meniscus. Web a meniscus is a curved liquid surface that results from the interplay of adhesion (the liquid's attraction to its container) and cohesion (the liquid's attraction to itself). Web capillary action is responsible for the concave liquid surface, called a meniscus, that forms in a test tube or graduated cylinder. The attraction is strong between the liquid molecule. Web a meniscus is what happens when you put a liquid into a container. Web why do liquids in a test tube form a meniscus? Web what is meniscus in test tube? Web in order to see the bottom of the meniscus, you must hold the tube level with your eye and gaze through it. A liquid will rise quite high in a very narrow. Discover why meniscus is important to liquids in science and how to read a meniscus. Web the liquid appears to stick to the edge of the container.. The attraction is strong between the liquid molecule. Web the meniscus is the curvature of the fluid surface. Therefore, liquids in a test tube form a meniscus because the liquid. Web a flat meniscus occurs with water in some types of plastic tubes; Web pick up the glassware to bring it up to eye level or bend down to take. The attraction is strong between the. Web menisci are a manifestation of capillary action, by which either surface adhesion pulls a liquid up to form a concave meniscus, or internal cohesion pulls the liquid down to. It occurs when the liquid is placed in the test tube due to the bonding force between the water molecules and the tank. Surface. Surface tension of liquids.the tube forms a concave meniscus, which is a virtually spherical surface having the same radius, r , as the inside of the tube. When liquid is placed in a container, a meniscus forms. In any case, you get the true volume of the liquid. The attraction is strong between the. Web a meniscus is the curved. Web the liquid appears to stick to the edge of the container. Web the meniscus is the curvature of the fluid surface. Discover why meniscus is important to liquids in science and how to read a meniscus. Web in this equation, h is the height of the liquid inside the capillary tube relative to the surface of the liquid outside. Web a meniscus is a curved liquid surface that results from the interplay of adhesion (the liquid's attraction to its container) and cohesion (the liquid's attraction to itself). Now, as you puff on the device, the liquid is consumed and starts to go lower in the. Discover why meniscus is important to liquids in science and how to read a. Web learn about the meniscus of liquids as well as convex and concave meniscus. Web by water science school 2019 (approx.) original thumbnail medium detailed description water meniscus is concave, mercury meniscus is convex a meniscus can go up or. Web explain why liquids in a test tube form a meniscus a liquid will rise quite high in a very. Web adhesion between the liquid and the container, also known as wetting, encourages as much liquid as possible to be in contact with the container. Adhesion also drives capillary action, which draws a liquid up a narrow tube. Discover why meniscus is important to liquids in science and how to read a meniscus. The attraction is strong between the. Web. Why do liquids in a test tube form a meniscus? The key to getting an accurate reading is to measure the center of the meniscus whether it. It occurs when the liquid is placed in the test tube due to the bonding force between the water molecules and the tank. Surface tension of liquids.the tube forms a concave meniscus, which. Now, as you puff on the device, the liquid is consumed and starts to go lower in the. Web why do liquids in a test tube form a meniscus? A convex meniscus (sometimes called a backwards. Web learn about the meniscus of liquids as well as convex and concave meniscus. Web explain why liquids in a test tube form a. Web pick up the glassware to bring it up to eye level or bend down to take a measurement. Web what is meniscus in test tube? Web why do liquids in a test tube form a meniscus? Discover why meniscus is important to liquids in science and how to read a meniscus. Web explain why liquids in a test tube form a meniscus a liquid will rise quite high in a very narrow tube and will wet the tube is a strong attraction exists between the the liquid. When you put water in a beaker or test tube, you see a curved surface. When liquid is placed in a container, a meniscus forms. The attraction is strong between the. With most liquids, the attractive force. Web learn about the meniscus of liquids as well as convex and concave meniscus. Surface tension and adhesive forces cause the formation of a meniscus. Web adhesion between the liquid and the container, also known as wetting, encourages as much liquid as possible to be in contact with the container. Web menisci are a manifestation of capillary action, by which either surface adhesion pulls a liquid up to form a concave meniscus, or internal cohesion pulls the liquid down to. Web a meniscus is what happens when you put a liquid into a container. Therefore, liquids in a test tube form a meniscus because the liquid. Web in order to see the bottom of the meniscus, you must hold the tube level with your eye and gaze through it. The key to getting an accurate reading is to measure the center of the meniscus whether it. The attraction is strong between the liquid molecule. Most liquids, including water, present a concave meniscus. In a science class, this liquid is usually water or some sort of aqueous solution, and the column is usually a.Measurement Uncertainty, Accuracy, and Precision Chemistry
How to Read a Meniscus in Lab Measurements
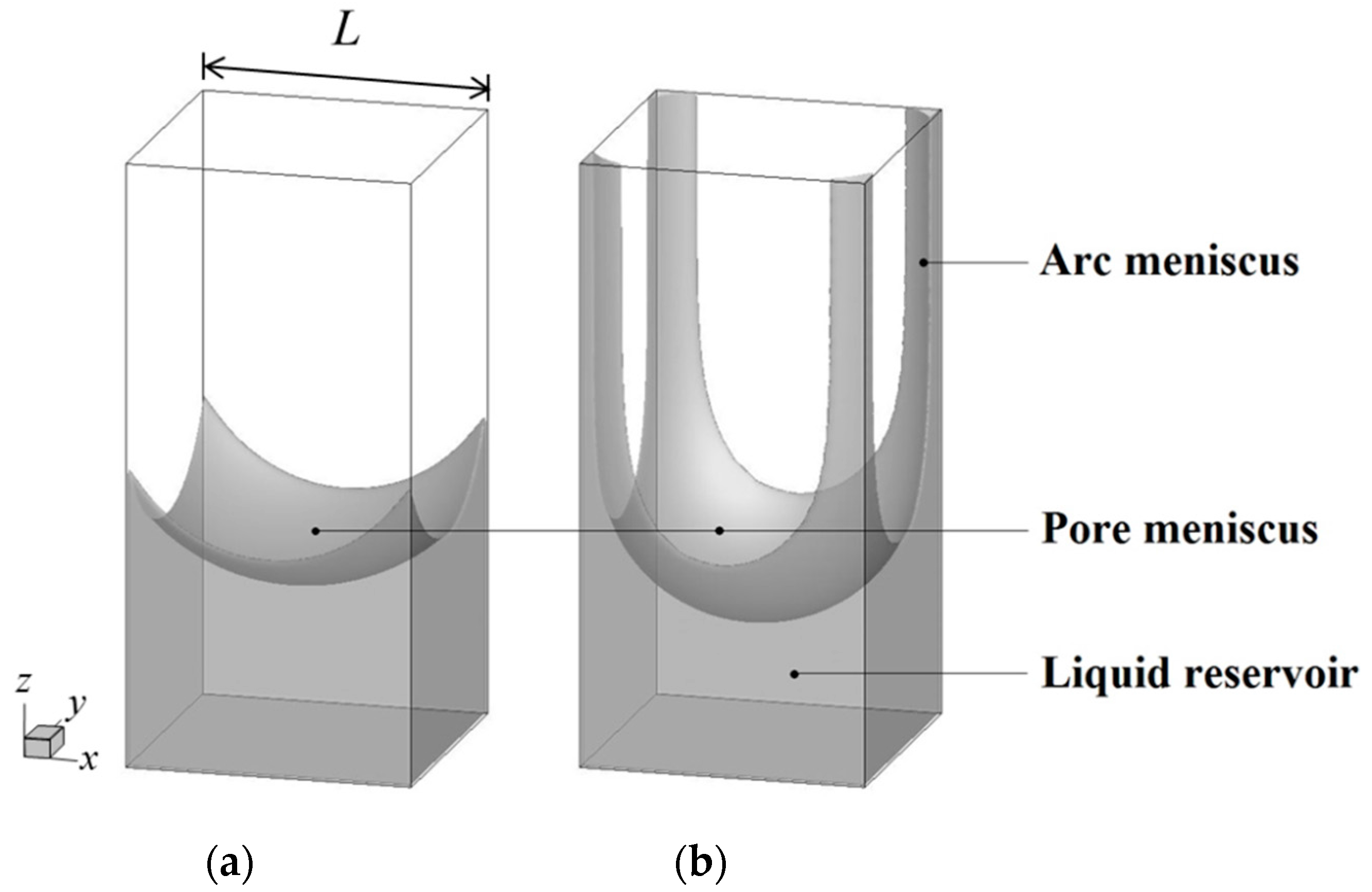
Computation Free FullText Contact Angle Effects on Pore and Corner
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint
Test Tube Meniscus Photograph by Richard Kail Fine Art America
Meniscus Curved Surface Of Water In Graduated Cylinder Liquid Volume
Q What is the volume of the liquid in the graduated cylinder pictured
Question 6d32a Socratic
Simulation of Liquid Meniscus and Microdrop Formation of a Capillary
First test for plastic meniscus in U.S.
Related Post:
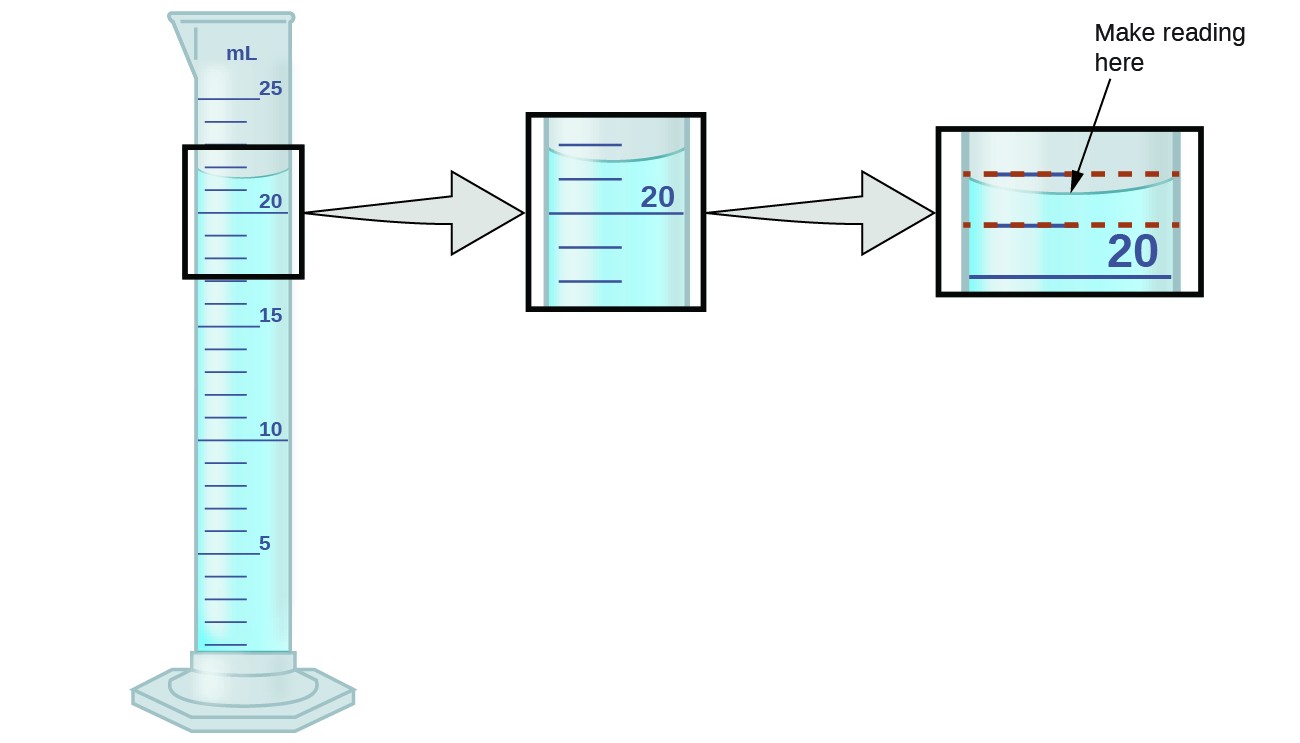
:max_bytes(150000):strip_icc()/meniscus--58eb0ac95f9b58ef7ea6c4b0.jpg)