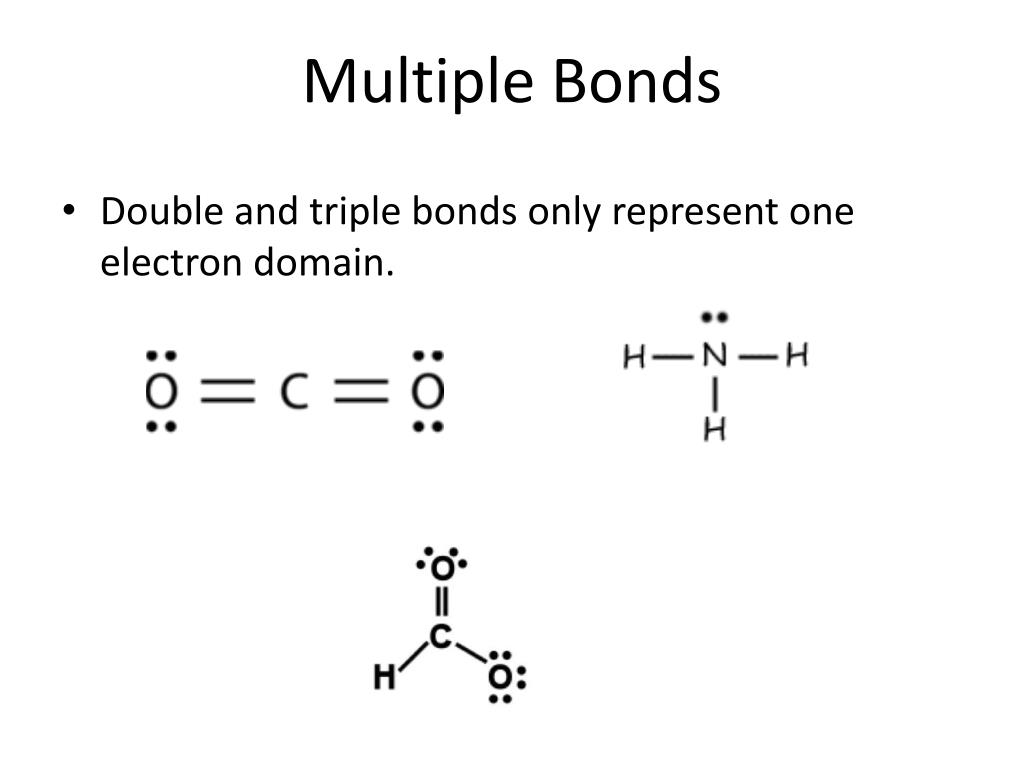
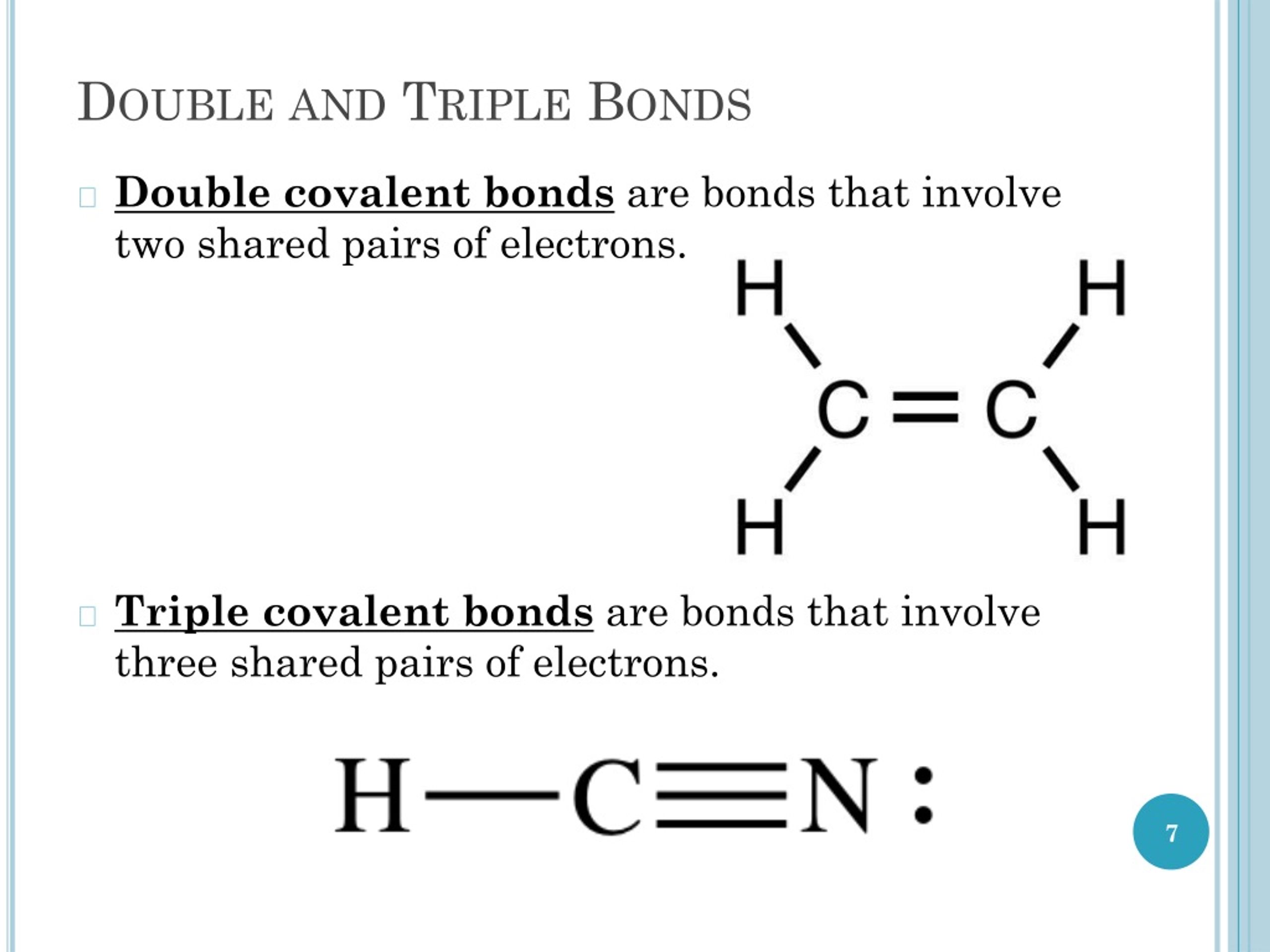
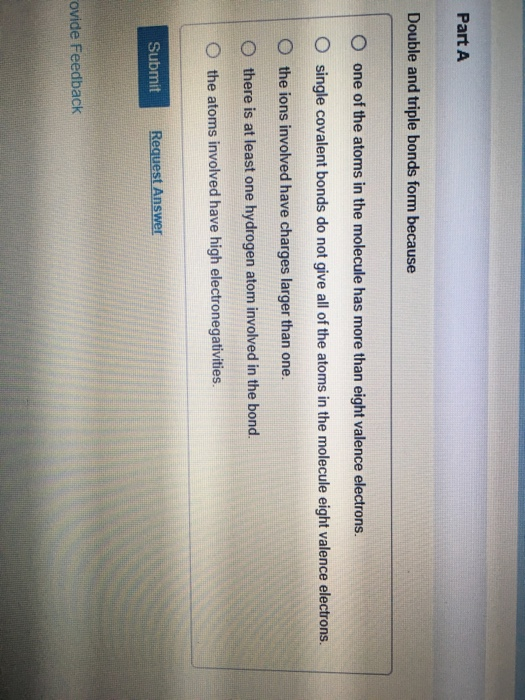
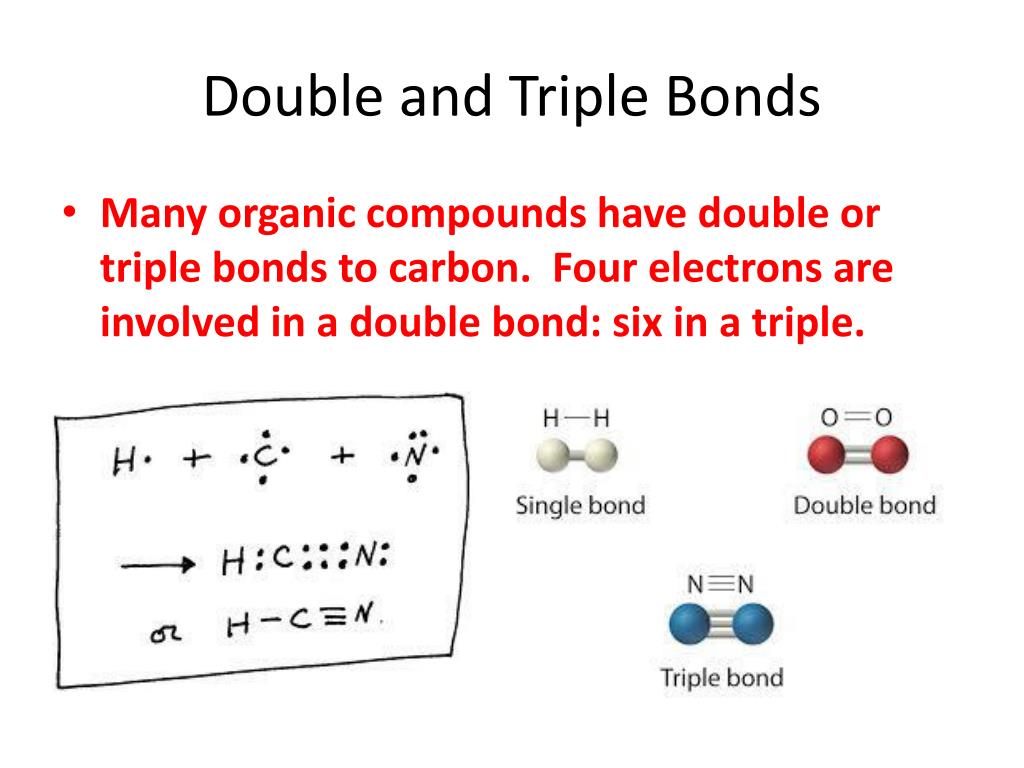
Double And Triple Bonds Form Because
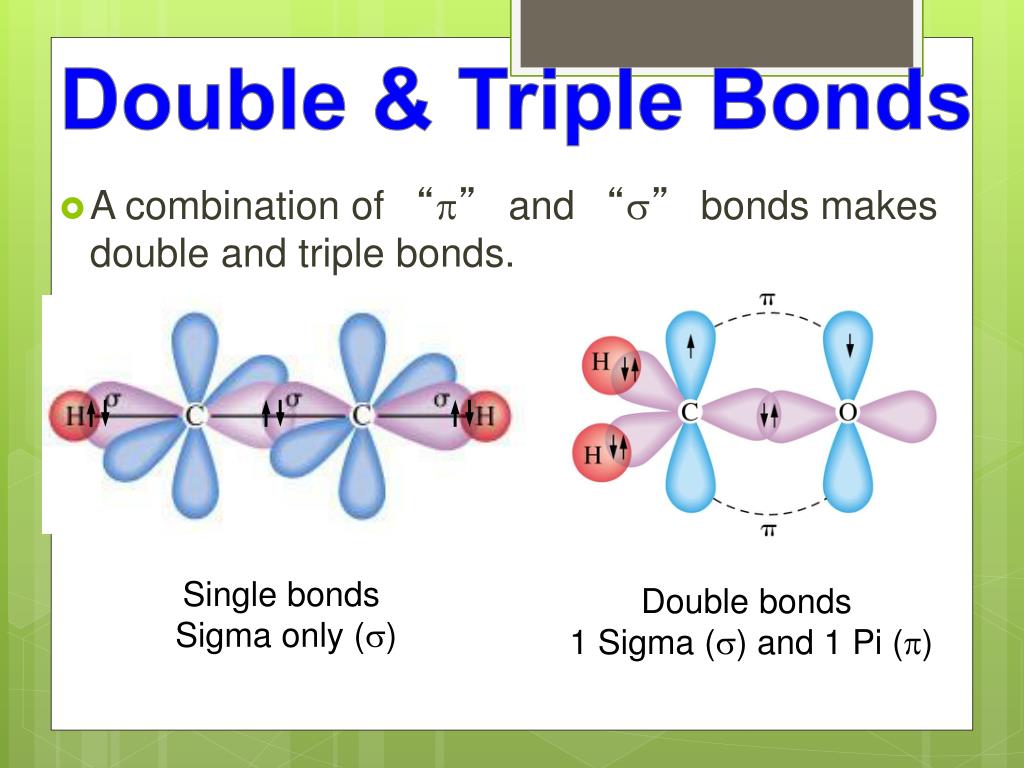
Double And Triple Bonds Form Because - The combination of \simga \simga and π π bonding orbitals produces a double. Web however, this would be extraordinarily rare and has just been discovered recently, so for almost all purposes, stick with a single, double, or triple bond for carbon. Web study with quizlet and memorize flashcards containing terms like how many electrons will magnesium gain or lose when it forms an ion? A double bond indicates sp 2 hybridization. Double and triple bonds form because. Click the card to flip 👆. Web in π π orbitals, the electron density lies above and below the axis connecting the bonded atoms. Web a double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in ch 2 o (formaldehyde) and between the two. 5.0 (2 reviews) ) double and triple bonds form because. Web choose the correct answer: 5.0 (2 reviews) ) double and triple bonds form because. Only single bonds bond indicates sp 3 hybridization. The seven diatomic molecules are: The atoms involved have high electronegativities b. Double and triple bonds form because: Web answered • expert verified. All of them are single pairing of electrons, but when the same atom forms multiple bonds. Multiple bonds consist of a σ bond located along the axis between two atoms and one or two π bonds. A triple bond indicates sp. Web choose the correct answer: Web choose the correct answer: Web a double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in ch 2 o (formaldehyde) and between the two. The σ bonds are usually formed by the. Web however, this would be extraordinarily rare and has just been discovered recently, so. Web why do some elements form double and triple bonds during bonding? Web a double bond is produced if two shared pairs of electrons exist between the same pair of atoms, and a triple bond is created by pairing three sets of electrons. 5.0 (2 reviews) ) double and triple bonds form because. Single covalent bonds do not give all. One of the atoms in the molecule has more than eight valence electrons. Web figure 8.3.4 8.3. A triple bond indicates sp. The atoms involved have high electronegativities b. Web we would like to show you a description here but the site won’t allow us. All bonds form as interactions of valence electrons of elements. The atoms involved have high electronegativities b. Single covalent bonds do not give all of the atoms in the molecule eight valence. Double and triple bonds form because: The combination of \simga \simga and π π bonding orbitals produces a double. All of them are single pairing of electrons, but when the same atom forms multiple bonds. Question 8 double and triple bonds form because a. A triple bond indicates sp. Web a double bond is produced if two shared pairs of electrons exist between the same pair of atoms, and a triple bond is created by pairing three sets of. Web double and triple bonds form because carbon which of the following elements does not exist as a diatomic molecule. Web figure 8.3.4 8.3. All bonds form as interactions of valence electrons of elements. Multiple bonds consist of a σ bond located along the axis between two atoms and one or two π bonds. The elements form multiple bonds because. All bonds form as interactions of valence electrons of elements. Web a double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in ch 2 o (formaldehyde) and between the two. Single covalent bonds do not give all of the atoms in the molecule eight valence. One of. Web we would like to show you a description here but the site won’t allow us. Click the card to flip 👆. Only single bonds bond indicates sp 3 hybridization. Web figure 8.3.4 8.3. Web however, this would be extraordinarily rare and has just been discovered recently, so for almost all purposes, stick with a single, double, or triple bond. Web double and triple bonds form because carbon which of the following elements does not exist as a diatomic molecule. A double bond indicates sp 2 hybridization. Double and triple bonds form because: Web we would like to show you a description here but the site won’t allow us. The σ bonds are usually formed by the. Web a double bond is produced if two shared pairs of electrons exist between the same pair of atoms, and a triple bond is created by pairing three sets of electrons. Web why do some elements form double and triple bonds during bonding? Single covalent bonds do not give all of the atoms in the molecule eight valence. Web in π π orbitals, the electron density lies above and below the axis connecting the bonded atoms. Click the card to flip 👆. Web study with quizlet and memorize flashcards containing terms like how many electrons will magnesium gain or lose when it forms an ion? Only single bonds bond indicates sp 3 hybridization. Web choose the correct answer: Web figure 8.3.4 8.3. Double and triple bonds form because. Multiple bonds consist of a σ bond located along the axis between two atoms and one or two π bonds. Web however, this would be extraordinarily rare and has just been discovered recently, so for almost all purposes, stick with a single, double, or triple bond for carbon. Question 8 double and triple bonds form because a. A triple bond indicates sp. All bonds form as interactions of valence electrons of elements.Multiple Bonds — Double & Triple Bonds Expii
PPT Chapter 9 Molecular Geometry and Bonding Theories PowerPoint
Multiple Bonds — Double & Triple Bonds Expii
Covalent Bonding (PART 1) Using the same nonmetal. ️ Single, double
Multiple Bonds — Double & Triple Bonds Expii
PPT Chapter 16 PowerPoint Presentation, free download ID636020
Organic compounds with both double and triple bonds YouTube
Solved Part A Double and triple bonds form because O one of
PPT Structure Determines Properties PowerPoint Presentation, free
PPT Valence Bond Theory PowerPoint Presentation, free download ID
Related Post: