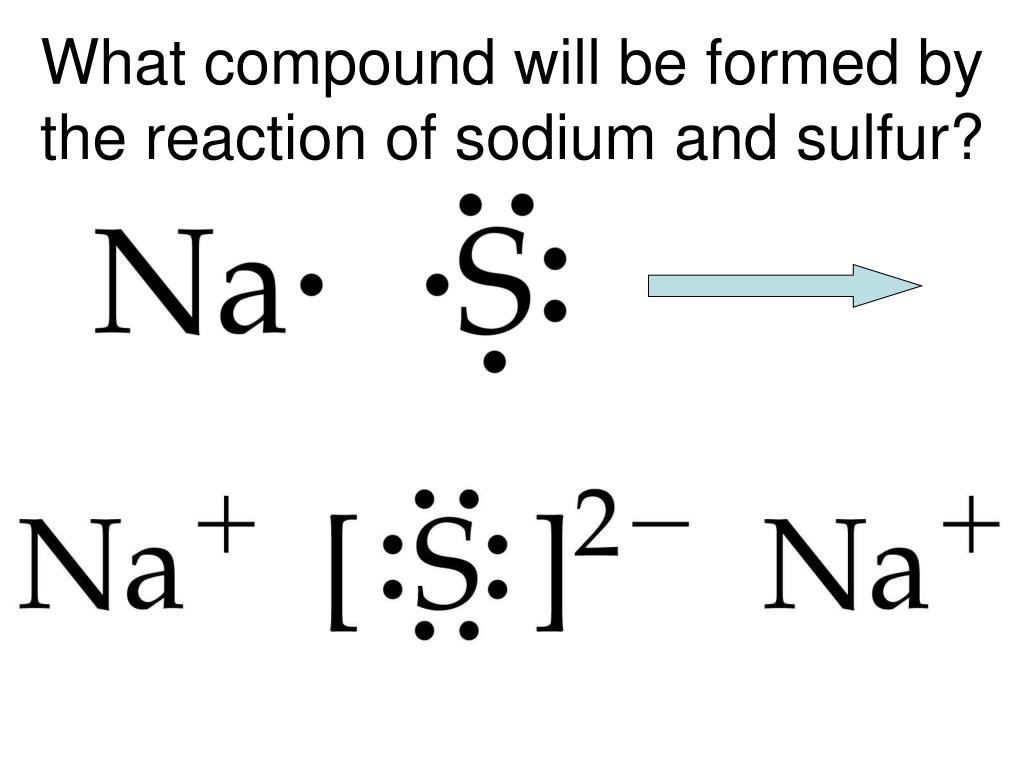Does Sulfur And Calcium Form An Ionic Compound
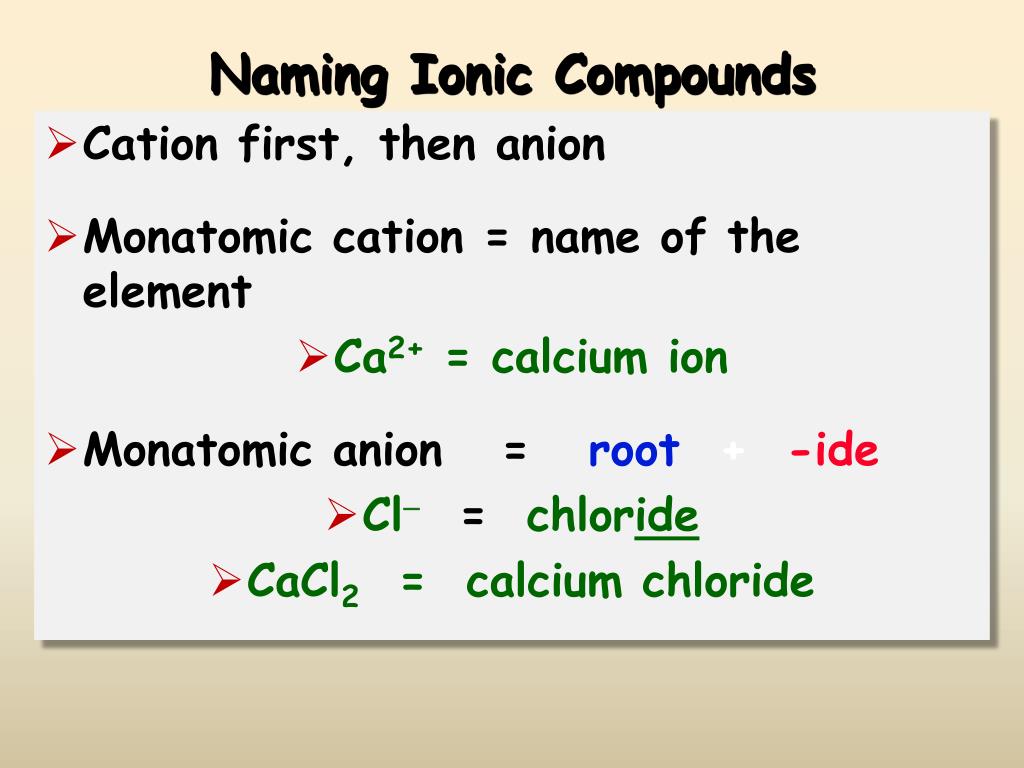
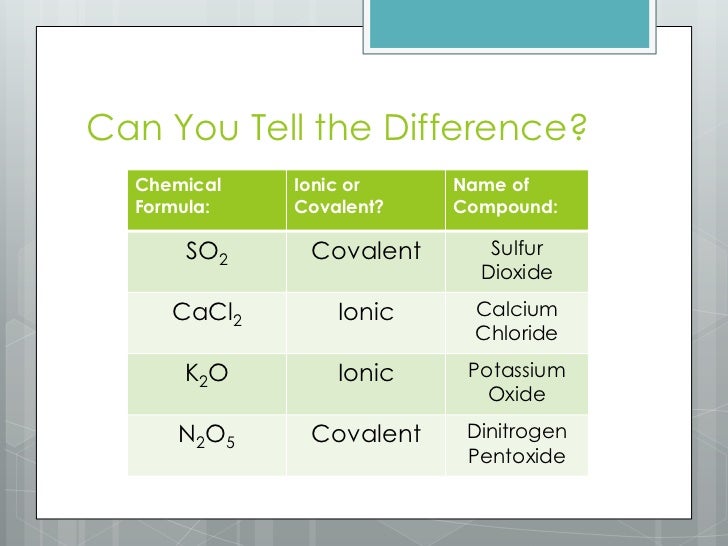
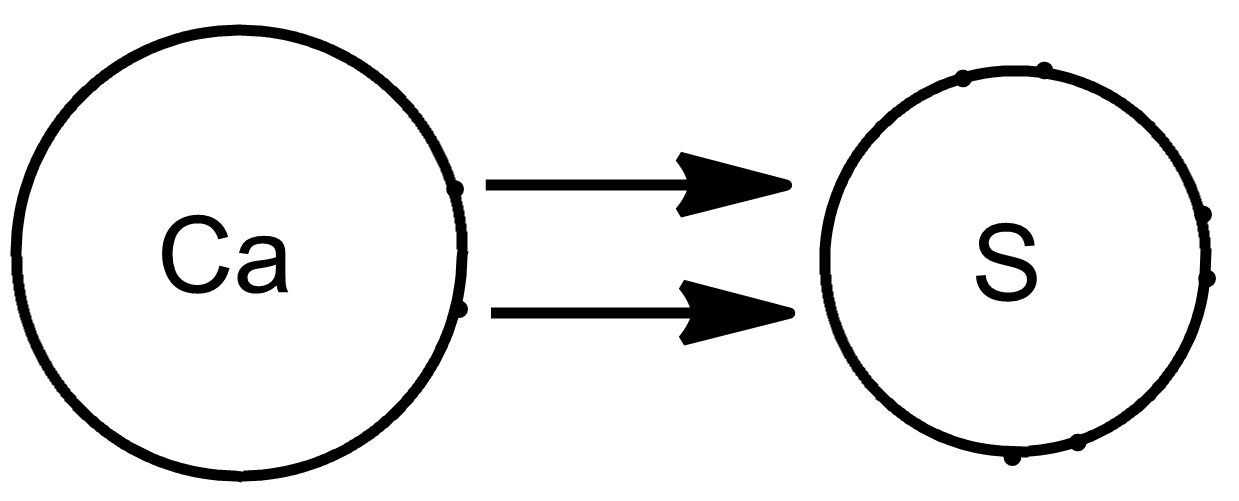
Does Sulfur And Calcium Form An Ionic Compound - Web and i know this is an ionic compound because calcium is a metal and sulfur is a non metal. And ionic compounds are formed when a metal and a nonmetal bond. The calcium ion and the oxygen ion; Based on the combinations listed in section 3.2, these. Web write the chemical formula for an ionic compound composed of each pair of ions. Web molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a molecule of the. A ca atom has two valence electrons. Web molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a. In the formula of an ionic compound we are showing the ratio. Elemental sulfur is a bright yellow crystalline solid at room temperature. Calcium commonly forms a cation with a charge of +2. Web sulfur and calcium can form an ionic compound, known as calcium sulfide (cas). In the ionic bond formation between calcium and sulfur, a calcium atom donates two. The calcium ion and the oxygen ion; Web by losing its 2 electrons calcium atom can achieve a noble gas configuration and. In the formula of an ionic compound we are showing the ratio. In the ionic bond formation between calcium and sulfur, a calcium atom donates two valence electrons to a sulfur atom to form a ca2+ ion and an s2+ ⁻ ion. The calcium ion and the oxygen ion; Web by losing its 2 electrons calcium atom can achieve a. Web what is the ionic bond formation of calcium and sulfur? A ca atom has two valence electrons. The calcium ion and the oxygen ion; Which element can replace x in the formula mg3x2? Calcium, which is in group (column) 2 on the periodic table, has a +2 ion charge. Web molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a. And ionic compounds are formed when a metal and a nonmetal bond. Web under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula s8. Which element can replace x in. A ca atom has two valence electrons. Element #1 element #2 forms ionic compound? When sulfur and calcium come into contact, a chemical reaction occurs,. Which element can replace x in the formula mg3x2? In the ionic bond formation between calcium and sulfur, a calcium atom donates two. Web in this example, the metal is the calcium and the nonmetal is the sulfur. Calcium, which is in group (column) 2 on the periodic table, has a +2 ion charge. Web under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula s8. The calcium ion and the oxygen ion; Calcium commonly forms a cation with a. Web in this example, the metal is the calcium and the nonmetal is the sulfur. Elemental sulfur is a bright yellow crystalline solid at room temperature. Web 6 years ago. The 2+ copper ion and the sulfur ion; Based on the combinations listed in section 3.2, these. Web write the chemical formula for the compound that is formed when aluminum and sulfur bond with one another. The 2+ copper ion and the sulfur ion; What is the correct formula for the ionic compound formed between calcium and sulfur? Calcium, which is in group (column) 2 on the periodic table, has a +2 ion charge. In the ionic. Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п. Web write the chemical formula for the compound that is formed when aluminum and sulfur bond with one another. Calcium, which is in group (column) 2 on the periodic table, has a +2 ion charge. Based on the combinations listed in section 3.2, these. Web by. The 2+ copper ion and the sulfur ion; Element #1 element #2 forms ionic compound? Web sulfur and calcium can form an ionic compound, known as calcium sulfide (cas). When sulfur and calcium come into contact, a chemical reaction occurs,. Web 6 years ago. The calcium ion and the oxygen ion; In the ionic bond formation between calcium and sulfur, a calcium atom donates two valence electrons to a sulfur atom to form a ca2+ ion and an s2+ ⁻ ion. And ionic compounds are formed when a metal and a nonmetal bond. Web write the chemical formula for an ionic compound composed of each pair of ions. Web 6 years ago. Web by losing its 2 electrons calcium atom can achieve a noble gas configuration and by gaining the 2 electrons sulfur atom can achieve noble gas configuration. Web molecular compounds can form compounds with different ratios of their elements, so prefixes are used to specify the numbers of atoms of each element in a. The calcium ion and the oxygen ion; Which element can replace x in the formula mg3x2? Web and i know this is an ionic compound because calcium is a metal and sulfur is a non metal. Calcium commonly forms a cation with a charge of +2. Web what is the ionic bond formation of calcium and sulfur? Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no п. Based on the combinations listed in section 3.2, these. Web write the chemical formula for an ionic compound composed of each pair of ions. In the formula of an ionic compound we are showing the ratio. Web sulfur and calcium can form an ionic compound, known as calcium sulfide (cas). The 2+ copper ion and the sulfur ion; Elemental sulfur is a bright yellow crystalline solid at room temperature. The 2+ copper ion and the sulfur ion;Solved What is the formula unit of the ionic compound that
Lewis Dot Diagram For Ca Hanenhuusholli
What Is An Ionic Compound? Formula and Defination
Which image depicts the initial atoms when calcium and sulfur form an
Ionic Bonding Presentation Chemistry
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint
PPT Molecules and Ions PowerPoint Presentation, free download ID
Bonding
PPT Ionic Bonds and Ionic Compounds PowerPoint Presentation, free
(a) Write the electron dot structure of calcium and sulphur.(b) Show
Related Post:




.PNG)