Does Carbon Form Ionic Bonds
Does Carbon Form Ionic Bonds - But it was not until 1985 that a new form of carbon was. Write the symbol for each. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web when electrons are transferred and ions form, ionic bonds result. Let’s consider both types of. Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between two atoms so that ions are formed. In most cases, carbon shares electrons with other atoms. More compounds of carbon exist than any other chemical element except for hydrogen. Web formation of ionic bonds involve removing electrons and there seems to be enough energy there. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Web there is only a small energy gap between the 2s and 2p orbitals, and so it pays the carbon to provide a small amount of energy to promote an electron from the 2s. Less commonly, carbon forms ionic bonds with other atoms. Web it has long been known that pure carbon occurs in different forms (allotropes) including graphite and. The textbook also mentions that a. Web carbon compounds are defined as chemical substances containing carbon. Less commonly, carbon forms ionic bonds with other atoms. Atoms interact with each other through the. Some atoms become more stable by gaining or losing an entire electron (or several electrons). Web aluminum and carbon react to form an ionic compound. When they do so, atoms form ions, or charged particles. Web carbon compounds are defined as chemical substances containing carbon. Ad browse & discover thousands of science book titles, for less. Web ions and ionic bonds. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web introduction to biological macromolecules. Web carbon compounds are defined as chemical substances containing carbon. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between. Web carbon compounds are defined as chemical substances containing carbon. Web there is only a small energy gap between the 2s and 2p orbitals, and so it pays the carbon to provide a small amount of energy to promote an electron from the 2s. Web ions and ionic bonds. So what's different for carbon? Let’s consider both types of. To form ionic bonds, carbon molecules must either gain or. Web there is only a small energy gap between the 2s and 2p orbitals, and so it pays the carbon to provide a small amount of energy to promote an electron from the 2s. Web carbon compounds are defined as chemical substances containing carbon. The most common type of bond. If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between. Web there is only a small energy gap between the 2s and 2p orbitals, and so it pays the carbon to provide a small amount of energy to promote. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. To form ionic bonds, carbon molecules must either gain or. Write the symbol for each. Web formation of ionic bonds involve removing electrons and there seems to be enough energy there. Web ions and ionic bonds. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between. Web ions and ionic bonds. Ad browse & discover thousands of science book titles, for less. Sap‑3 (eu) , sap‑3.a (lo). In most cases, carbon shares electrons with other atoms. The textbook also mentions that a. Web introduction to biological macromolecules. Let’s consider both types of. Ad browse & discover thousands of science book titles, for less. Web aluminum and carbon react to form an ionic compound. Some atoms become more stable by gaining or losing an entire electron (or several electrons). Ad browse & discover thousands of science book titles, for less. Predict which forms an anion, which forms a cation, and the charges of each ion. More compounds of carbon exist than any other chemical element except for hydrogen. Atoms interact with each other through the. Write the symbol for each. When they do so, atoms form ions, or charged particles. The most common type of bond formed by carbon is a covalent bond. If the bond is with another carbon atom, it is a pure covalent (or nonpolar covalent) bond. Web carbon compounds are defined as chemical substances containing carbon. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web ions and ionic bonds. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. But it was not until 1985 that a new form of carbon was. Carbon’s ability to form bonds with four other atoms goes back to its number and configuration of electrons. Let’s consider both types of. Sap‑3 (eu) , sap‑3.a (lo) google classroom. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. This is because carbon has 4 valence.Carbon to Carbon Single, Double & Triple Bonds Surfguppy
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
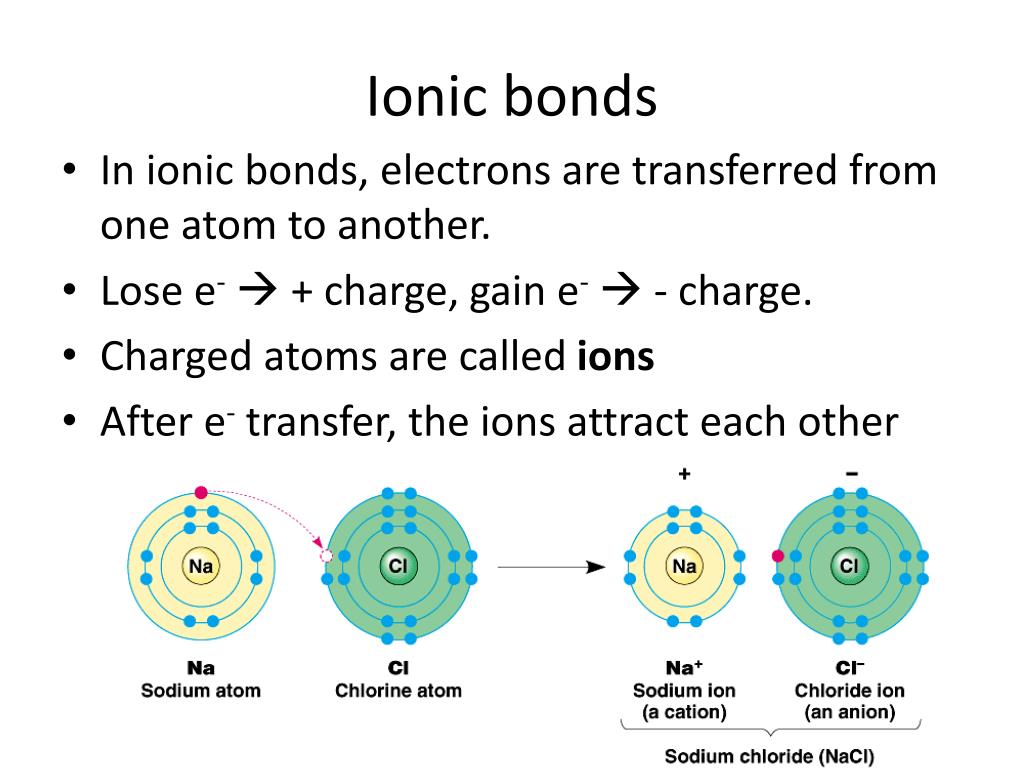
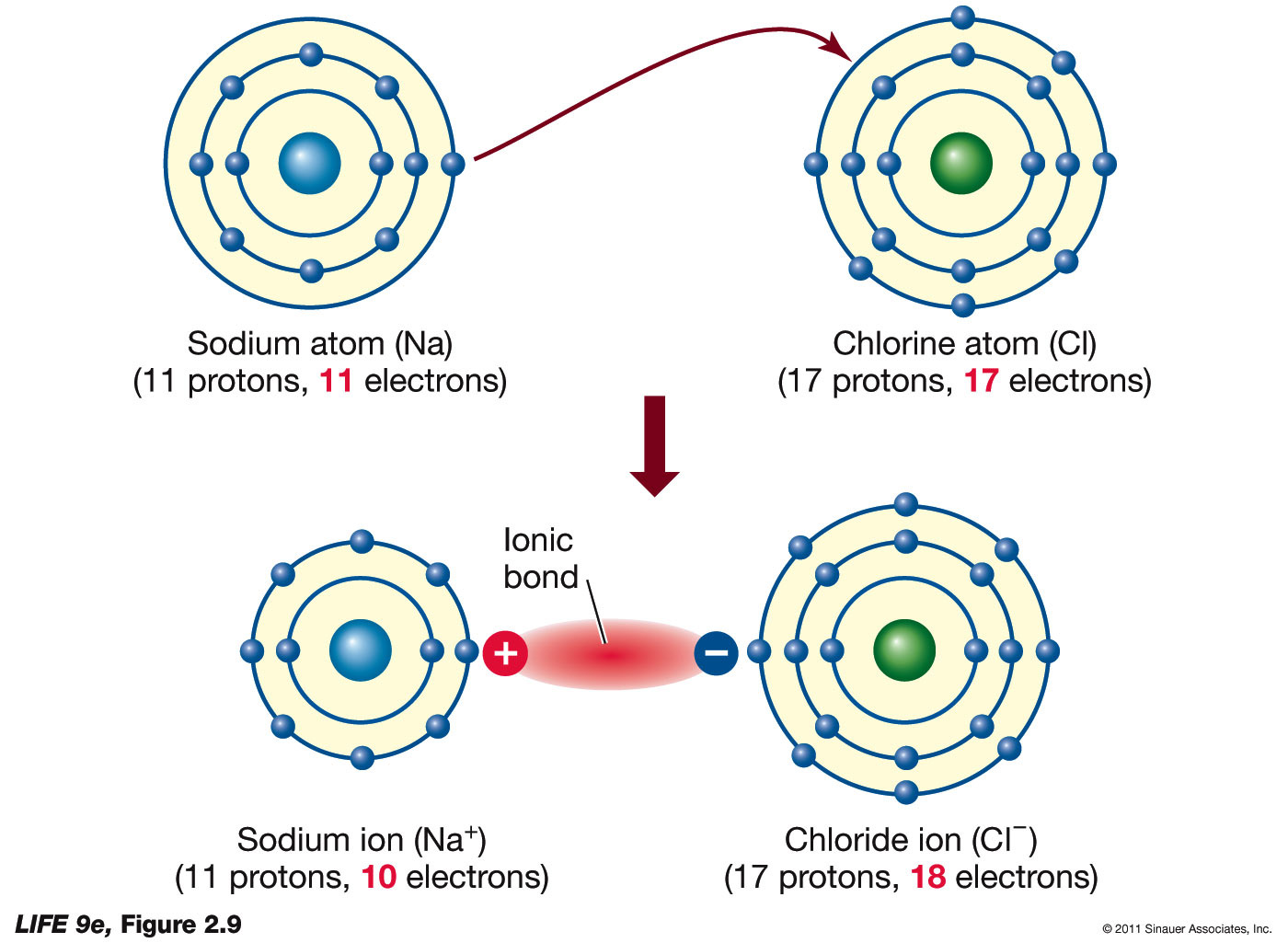
Ionic Bond Definition, Types, Properties & Examples
Ppt Atomic Structure And Chemical Bonding Powerpoint Presentation 7F5
Ionic Bond Definition, Types, Properties & Examples
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic Bonding Presentation Chemistry
Four covalent bonds. Carbon has four valence electrons and here a
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
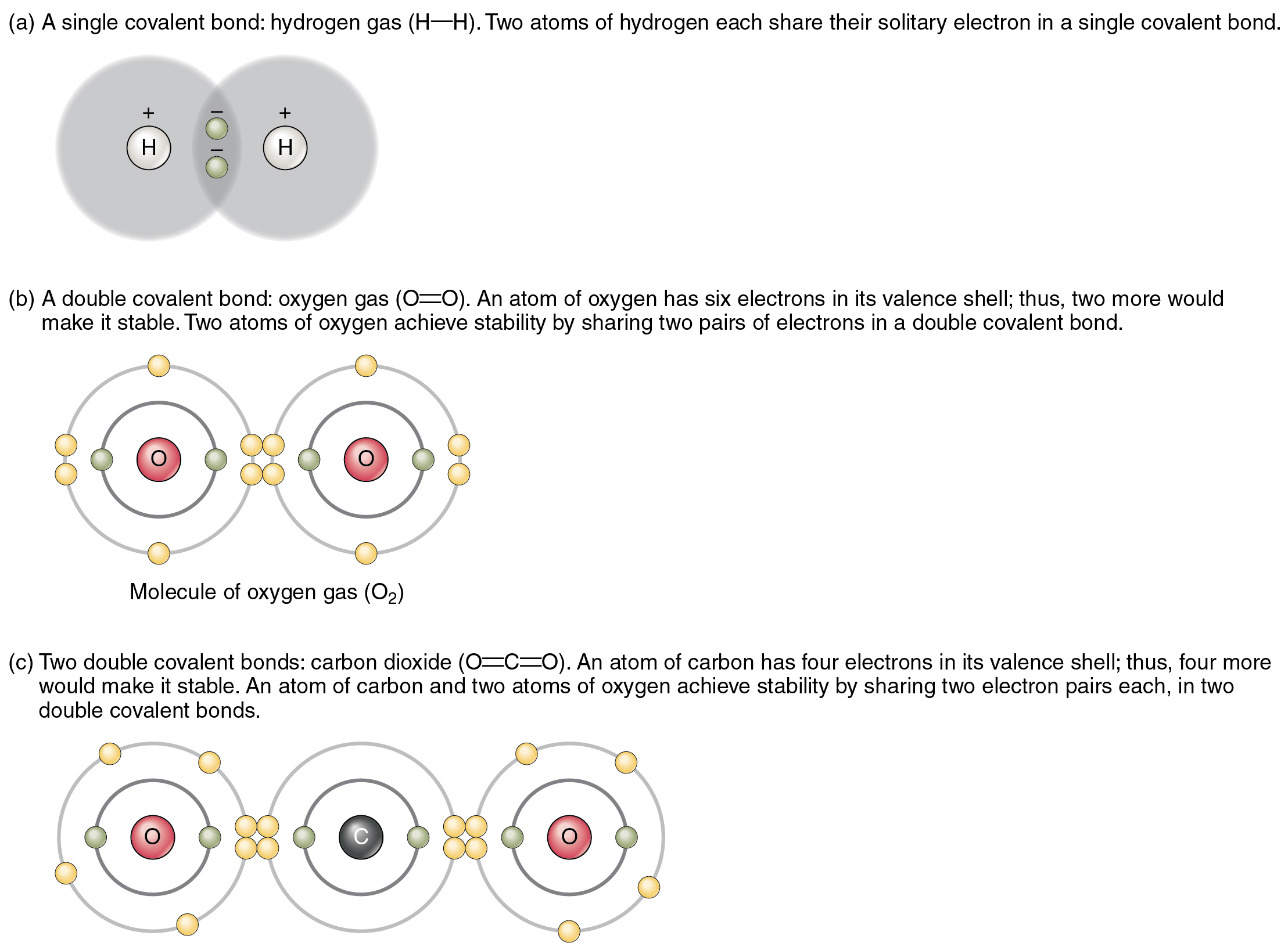
Chemical Bonds · Anatomy and Physiology
Related Post:




.PNG)