Covalent Bonds Form When 2 Atoms Blank Electrons
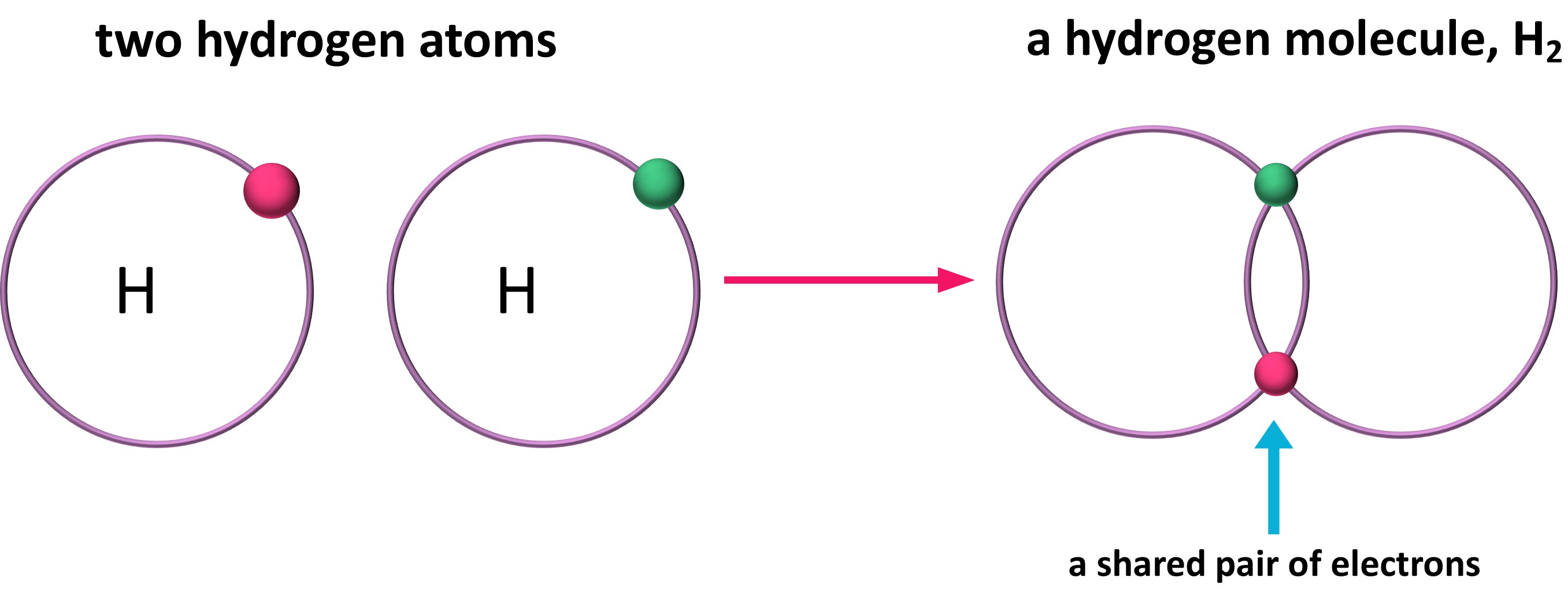
Covalent Bonds Form When 2 Atoms Blank Electrons - Chemists frequently use lewis diagrams to represent covalent bonding. This gives 4 + (3 × 6) + 2 = 24 valence electrons. Web covalent bonds where electrons are not shared equally between two atoms are called polar covalent bond. Web two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. The number of bonds an element forms in a covalent compound is determined by the. A covalent bond is a chemical bond that involves the sharing of electrons to form. We refer to this as a. As the atoms collide with each other, some of the energy involved in the collision is usually released to the. Nitrogen atoms will form three. Web a single covalent bond is when two atoms share a single pair of. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. A covalent bond is a chemical bond that involves the sharing of electrons to form. Introduction living things are made up of atoms, but in most cases,. Web a covalent bond forming h 2 (right) where two hydrogen. A (n) ____ bond involves the sharing of electron pairs between atoms, also known as a molecular bond. The potential energy of two separate hydrogen. Nitrogen atoms will form three. Introduction living things are made up of atoms, but in most cases,. Energy is always released when bonds are made. A covalent bond is a chemical bond that involves the sharing of electrons to form. Web if the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared equally. The number of bonds an element forms in a covalent compound is determined by. Web the bond length is determined by the distance at which the lowest potential energy is achieved. Web when electrons are shared between two atoms, they make a bond called a covalent bond. Web a covalent bond is formed between two atoms by sharing electrons. Web a bond in which the electronegativity difference between the atoms is between 0.5 and. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. In covalent bonds, electrons are shared between the. Web covalent bonds where electrons are not shared equally between two atoms are called polar covalent bond. Web types of chemical bonds including covalent, ionic,. A (n) ____ bond involves the sharing of electron pairs between atoms, also known as a molecular bond. Chemists frequently use lewis diagrams to represent covalent bonding. Web created by lydiascroggs terms in this set (21) in ionic bonds, the electrons are transferred from the metal to the nonmetal. Web for a covalent bond to form, we need two atoms. When one pair of electrons is shared between two atoms,. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Chemists frequently use lewis diagrams to represent covalent bonding. A (n) ____ bond involves the sharing of electron pairs between atoms, also known as a molecular bond. Two. Web if the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared equally. Introduction living things are made up of atoms, but in most cases,. We refer to this as a. Nitrogen atoms will form three. When atoms of different elements share. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. In covalent bonds, electrons are shared between the. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more. A (n) ____ bond involves the sharing of electron. Chemists frequently use lewis diagrams to represent covalent bonding. When one pair of electrons is shared between two atoms,. Example of a polar covalent bond. Energy is always released when bonds are made. Web a single covalent bond is when two atoms share a single pair of. Nitrogen atoms will form three. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar. Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons. In covalent bonds, electrons are shared between the. A polar covalent bond is a. Introduction living things are made up of atoms, but in most cases,. Web the covalent bond atoms can combine to achieve an octet of valence electrons by sharing electrons. Web two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. Web covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or. Example of a polar covalent bond. A (n) ____ bond involves the sharing of electron pairs between atoms, also known as a molecular bond. Web web hydrogen the atomic number of sulfur is 16. The potential energy of two separate hydrogen. Web only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. Chemists frequently use lewis diagrams to represent covalent bonding. The number of bonds an element forms in a covalent compound is determined by the number of. As the atoms collide with each other, some of the energy involved in the collision is usually released to the. Web molecular orbital the orbital of the shared electrons a covalent bond forms when the attractive and repulsive forces between two atoms are balanced. Energy is always released when bonds are made.Covalent bonding in an oxygen molecule. Covalent bonding, Chemistry
Covalent Bond Definition, Types, and Examples
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Anatomy and Physiology Chemical Bonds
Covalent Bonding (Biology) — Definition & Role Expii
Double Covalent Bond Definition and Examples
Covalent Bond sharing of electrons between atoms; bonds contain energy
covalent bond Definition, Properties, Examples, & Facts Britannica
covalent bond definition, properties and examples The Science Core
Related Post: