Can P Orbitals Form Sigma Bonds
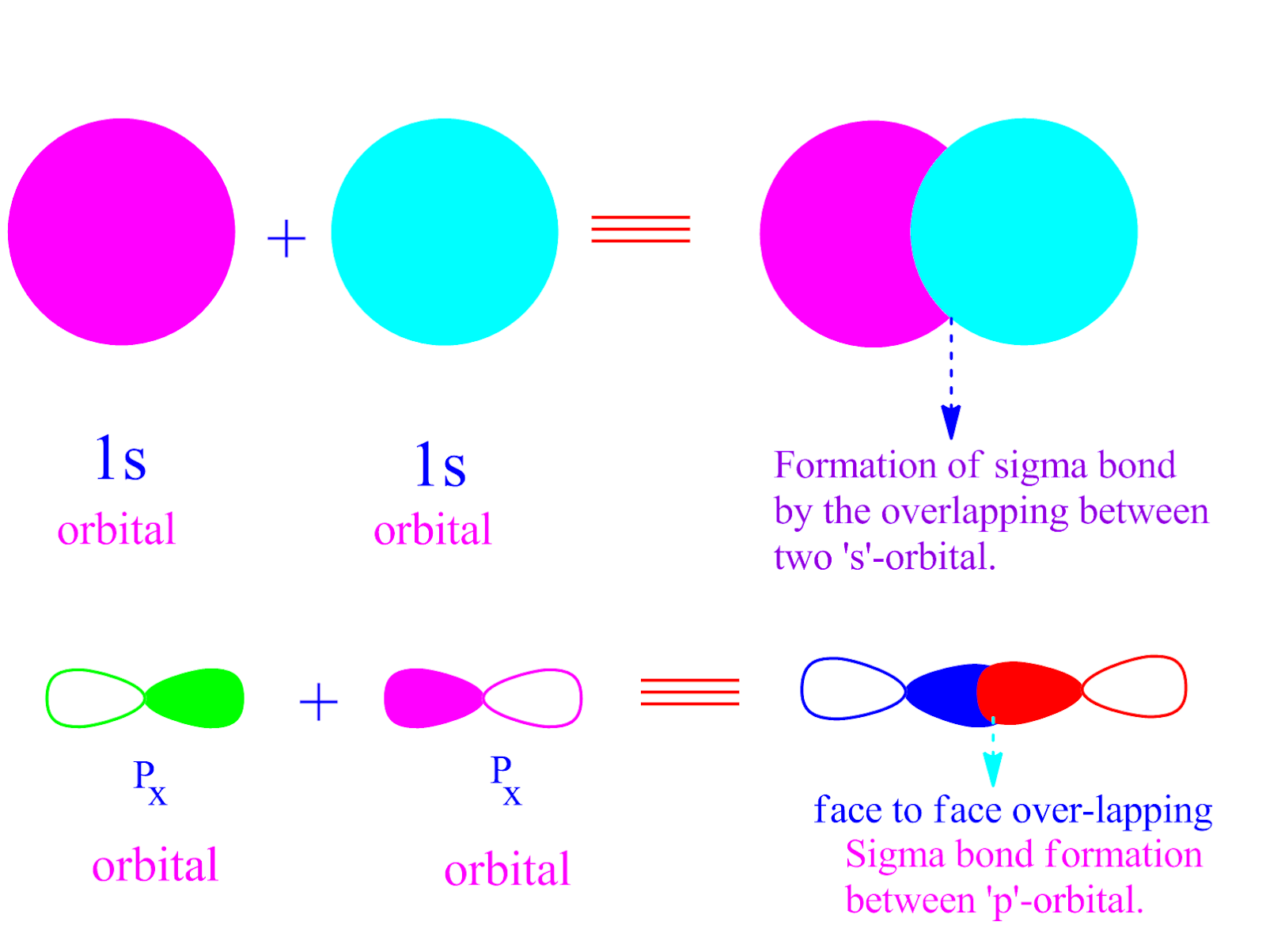
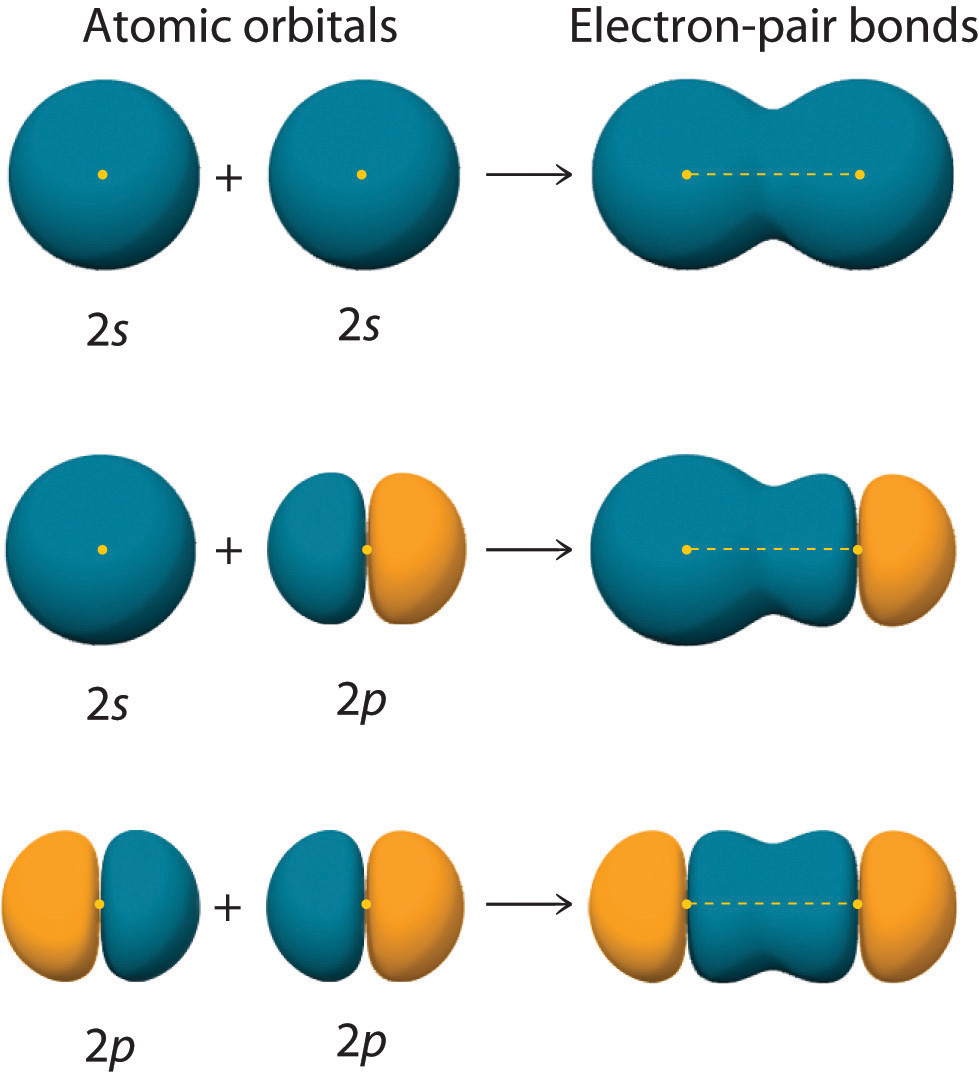
Can P Orbitals Form Sigma Bonds - A sigma bond σ is the strongest type of covalent bond in which the atomic orbitals directly. Web 11 years ago. Illustration of a pi bond forming from two p orbitals. Web there are three possible atomic orbitals in the 2p level where some of these electrons could be found: Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. Web pi bonds (\pi) (π) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Hydrogen fluoride (hf) is an example: Pi bonds are usually weaker than sigma bonds. P x, p y and p z. Web we would like to show you a description here but the site won’t allow us. P x, p y and p z. A p orbital lies along a particular axis: A sigma bond can also be formed by the overlap. Illustration of a pi bond forming from two p orbitals. Web sigma (σ) and pi (π) bonds form in covalent substances when atomic orbitals overlap. A sigma bond σ is the strongest type of covalent bond in which the atomic orbitals directly. Examples of orbitals with appropriate symmetry are the \(s\). A sigma bond can also be formed by the overlap. Pi bonds are usually weaker than sigma bonds. Web a sigma bond can be formed by overlap of an s atomic orbital with a. Web in considering the interaction of two p orbitals, we have to keep in mind that p orbitals are directional. And a hybridized orbital cannot be involved in a pi bond. Web 11 years ago. Illustration of a pi bond forming from two p orbitals. Web there are three possible atomic orbitals in the 2p level where some of these. A p orbital lies along a particular axis: Web sigma (σ) and pi (π) bonds form in covalent substances when atomic orbitals overlap. Ad browse & discover thousands of science book titles, for less. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. A sigma bond σ is the strongest. A p orbital lies along a particular axis: Web pi bonds (\pi) (π) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. The first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. And a hybridized orbital cannot be involved in a pi bond. Web \(d\). P x, p y and p z. A sigma bond can also be formed by the overlap. Web there are three possible atomic orbitals in the 2p level where some of these electrons could be found: Illustration of a pi bond forming from two p orbitals. Web we would like to show you a description here but the site won’t. Hydrogen fluoride (hf) is an example: There will be both bonding and antibonding combinations. Web \(d\) orbitals can also form \(\sigma\) bonds with other types of orbitals with the appropriate symmetry. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. Web 11 years ago. Web the two p orbitals orthogonal to the bond axis can engage in p bonding. Web 11 years ago. Web in considering the interaction of two p orbitals, we have to keep in mind that p orbitals are directional. A sigma bond can also be formed by the overlap. Hydrogen fluoride (hf) is an example: Illustration of a pi bond forming from two p orbitals. Web the two p orbitals orthogonal to the bond axis can engage in p bonding. Ad browse & discover thousands of science book titles, for less. We need to look at the interaction between the s and p x, p y and p z orbitals on one nitrogen atom with. Web \(d\) orbitals can also form \(\sigma\) bonds with other types of orbitals with the appropriate symmetry. Ad browse & discover thousands of science book titles, for less. We need to look at the interaction between the s and p x, p y and p z orbitals on one nitrogen atom with the s and p x, p y and. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. P x, p y and p z. A sigma bond σ is the strongest type of covalent bond in which the atomic orbitals directly. There will be both bonding and antibonding combinations. Web we would like to show you a description here but the site won’t allow us. Web \(d\) orbitals can also form \(\sigma\) bonds with other types of orbitals with the appropriate symmetry. A p orbital lies along a particular axis: Examples of orbitals with appropriate symmetry are the \(s\). Web pi bonds (\pi) (π) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Web 11 years ago. Possible orbital combinations to generate sigma. We need to look at the interaction between the s and p x, p y and p z orbitals on one nitrogen atom with the s and p x, p y and p z orbitals on the other. Web the two p orbitals orthogonal to the bond axis can engage in p bonding. Pi bonds are usually weaker than sigma bonds. Web in considering the interaction of two p orbitals, we have to keep in mind that p orbitals are directional. Web there are three possible atomic orbitals in the 2p level where some of these electrons could be found: A sigma bond can also be formed by the overlap. Illustration of a pi bond forming from two p orbitals. And a hybridized orbital cannot be involved in a pi bond. Hydrogen fluoride (hf) is an example:Sigma and Pi Bonds — Definition & Overview Expii
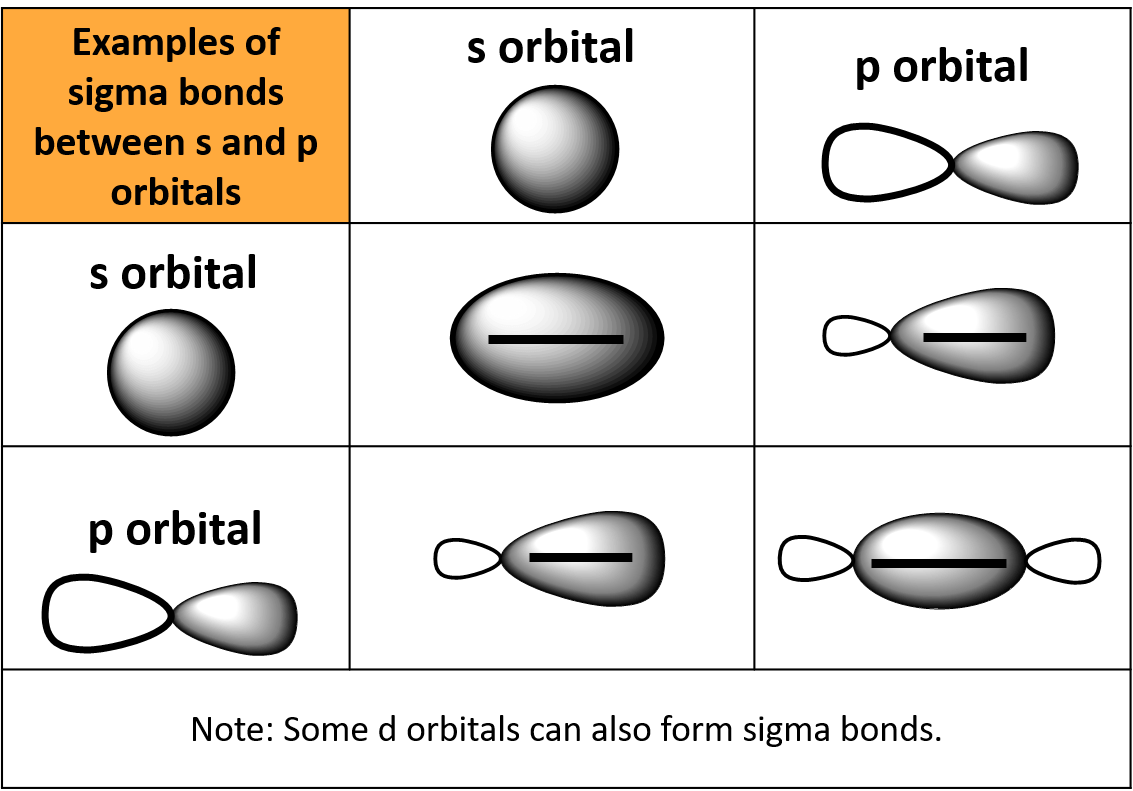
Can a s orbital overlap with any p orbital to form a sigma bond
[Solved] sketch sigma and pi bond from p orbital Course Hero
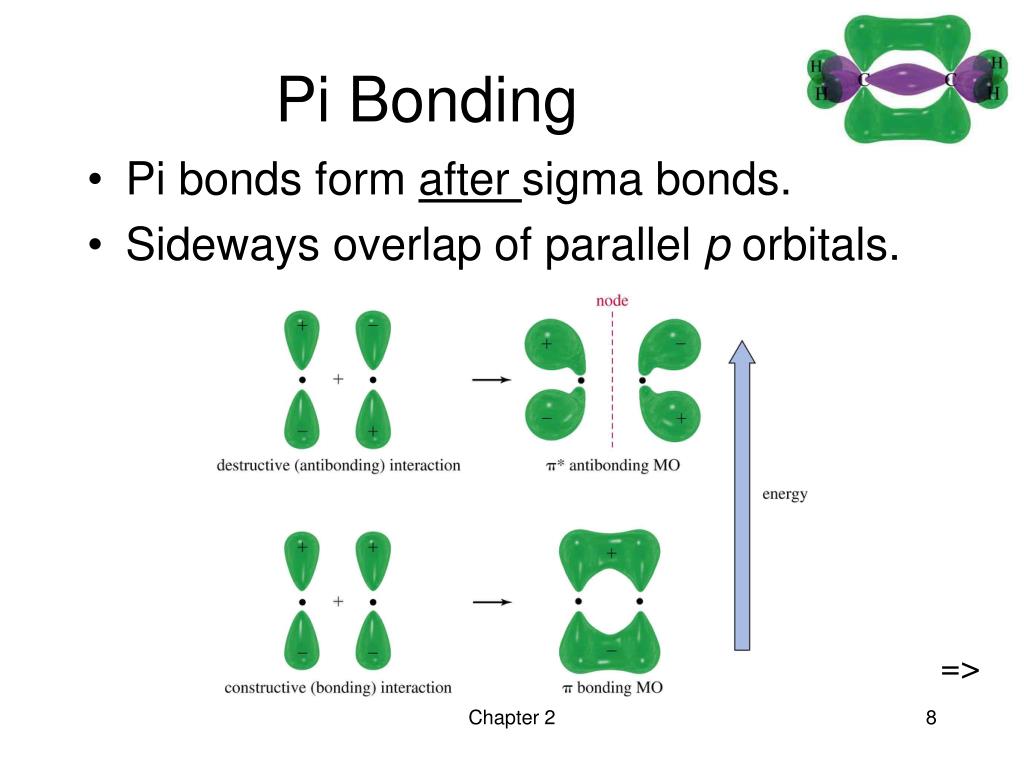
PPT Chapter 2 Structure and Properties of Organic Molecules
PPT Sigma and Pi Bonding PowerPoint Presentation, free download ID
Sigma and Pi Bonds Brilliant Math & Science Wiki
Chapters 9 and 11 study guide
Sigma and Pi Bonds — Definition & Overview Expii
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts
Related Post: