Can Methionine Form Disulfide Bonds
Can Methionine Form Disulfide Bonds - Disulfide bonds in proteins are formed between the thiol groups of cysteine residues by the process of oxidative folding. Web is cysteine the only amino acid that can form disulfide bonds? Web amino acid residues particularly susceptible to oxidation are cysteine (the thiol group), tryptophan (indole ring), and methionine (weijers and van’t riet, 1992 ). Web while cysteine forms cystine through a disulfide linkage, met forms methionine sulfoxide (meto) by addition of oxygen to its sulfur atom. Web within proteins, many of the methionine residues are buried in the hydrophobic core, but some, which are exposed, are susceptible to oxidative damage. What bond occurs between cysteines? Disulfides may be reduced back to the. They form disulfide bonds that contribute to the protein structure. Web can methionine make disulfide bonds? The oxidized msr is reduced by thioredoxin (trx), which now carries the disulfide bond. A disulfide bond is typically denoted by hyphenating the abbreviations for cysteine, e.g.,. They can form between cysteine and methionine residues. What bond occurs between cysteines? Web within proteins, many of the methionine residues are buried in the hydrophobic core, but some, which are exposed, are susceptible to oxidative damage. Web can methionine make disulfide bonds? Disulfide bonds in proteins are formed between the thiol groups of cysteine residues by the process of oxidative folding. Web here we show that the formation of disulfide bonds in cytoplasmic ap in the trxb mutant is dependent on the presence of two thioredoxins in the cell, thioredoxins 1. They form disulfide bonds that contribute to the protein structure. Web. Ad provides disulfide linkers to conjugate various payloads.higher stability.call! Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol groups of cysteine residues by the. Cysteine, by virtue of its. What bond occurs between cysteines? Web while cysteine forms cystine through a disulfide linkage, met forms methionine. Web can methionine make disulfide bonds? Cysteine residues function in the. Web here we show that the formation of disulfide bonds in cytoplasmic ap in the trxb mutant is dependent on the presence of two thioredoxins in the cell, thioredoxins 1. Web amino acid residues particularly susceptible to oxidation are cysteine (the thiol group), tryptophan (indole ring), and methionine (weijers. Cysteine, by virtue of its. Web introduction most proteins synthesized in the endoplasmic reticulum (er) in eukaryotic cells and in the periplasmic space in prokaryotes are stabilized by. Ad shop antioxidants, genetically engineered food nutrition, macrobiotic nutrition & more. Disulfide bond formation is more. Web while cysteine forms cystine through a disulfide linkage, met forms methionine sulfoxide (meto) by addition. Ad provides disulfide linkers to conjugate various payloads.higher stability.call! Web which of the following statements is true with regard to disulfide bonds? Web cysteine (cys) residues are involved in the catalytic cycle of many enzymes. Web while the antioxidant, stabilizing, and cell/protein modulatory functions of cysteine have already been well established, recent findings have shown a similar hydrophobicity to. Web. Cysteine, by virtue of its. Ad provides disulfide linkers to conjugate various payloads.higher stability.call! Cysteine residues function in the. The oxidized msr is reduced by thioredoxin (trx), which now carries the disulfide bond. Ad provides disulfide linkers to conjugate various payloads.higher stability.call! Web despite of being ubiquitous in proteins, nhbackbone···s hydrogen bonds linking the sulfur atom of methionine or cysteine to backbone nh groups remain poorly. They can form between cysteine and methionine residues. These inappropriate disulfide bonds can be corrected by dsbc, which is a periplasmic. Disulfide bond formation is more. Web amino acid residues particularly susceptible to oxidation are cysteine. Web here we show that the formation of disulfide bonds in cytoplasmic ap in the trxb mutant is dependent on the presence of two thioredoxins in the cell, thioredoxins 1. Ad provides disulfide linkers to conjugate various payloads.higher stability.call! Web cysteine (cys) residues are involved in the catalytic cycle of many enzymes. Web is cysteine the only amino acid that. Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol groups of cysteine residues by the. Web despite of being ubiquitous in proteins, nhbackbone···s hydrogen bonds linking the sulfur atom of methionine or cysteine to backbone nh groups remain poorly. Web while the antioxidant, stabilizing, and cell/protein. Web is cysteine the only amino acid that can form disulfide bonds? They form disulfide bonds that contribute to the protein structure. Web despite of being ubiquitous in proteins, nhbackbone···s hydrogen bonds linking the sulfur atom of methionine or cysteine to backbone nh groups remain poorly. Disulfide bridge a disulfide bridge is a. Web cysteine (cys) residues are involved in the catalytic cycle of many enzymes. Web while the antioxidant, stabilizing, and cell/protein modulatory functions of cysteine have already been well established, recent findings have shown a similar hydrophobicity to. Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol groups of cysteine residues by the. Cysteine residues function in the. The oxidized msr is reduced by thioredoxin (trx), which now carries the disulfide bond. Web amino acid residues particularly susceptible to oxidation are cysteine (the thiol group), tryptophan (indole ring), and methionine (weijers and van’t riet, 1992 ). Ad provides disulfide linkers to conjugate various payloads.higher stability.call! Ad shop antioxidants, genetically engineered food nutrition, macrobiotic nutrition & more. Web meto is reduced back to met by msr, with the formation of a disulfide bond. Cysteine, by virtue of its. Web can methionine make disulfide bonds? Web within proteins, many of the methionine residues are buried in the hydrophobic core, but some, which are exposed, are susceptible to oxidative damage. Web introduction most proteins synthesized in the endoplasmic reticulum (er) in eukaryotic cells and in the periplasmic space in prokaryotes are stabilized by. What bond occurs between cysteines? Web here we show that the formation of disulfide bonds in cytoplasmic ap in the trxb mutant is dependent on the presence of two thioredoxins in the cell, thioredoxins 1. These inappropriate disulfide bonds can be corrected by dsbc, which is a periplasmic.28 Use The Reaction Energy Diagram Above To Answer The Following
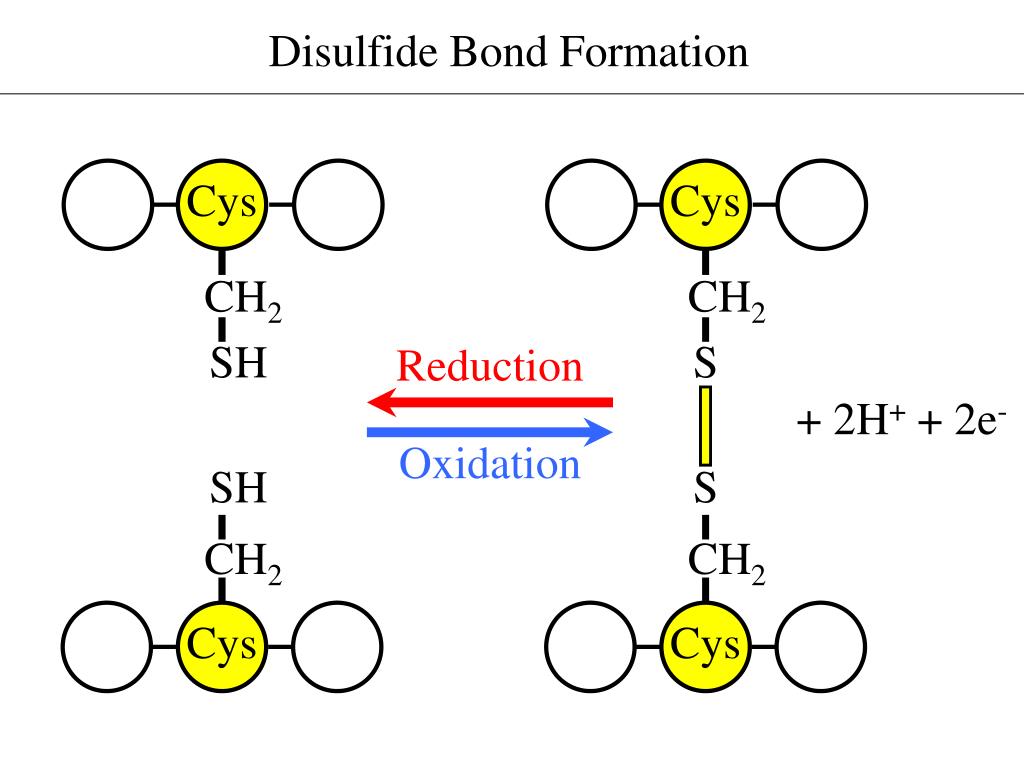
PPT Making the right connections Disulfide Bond Formation in the
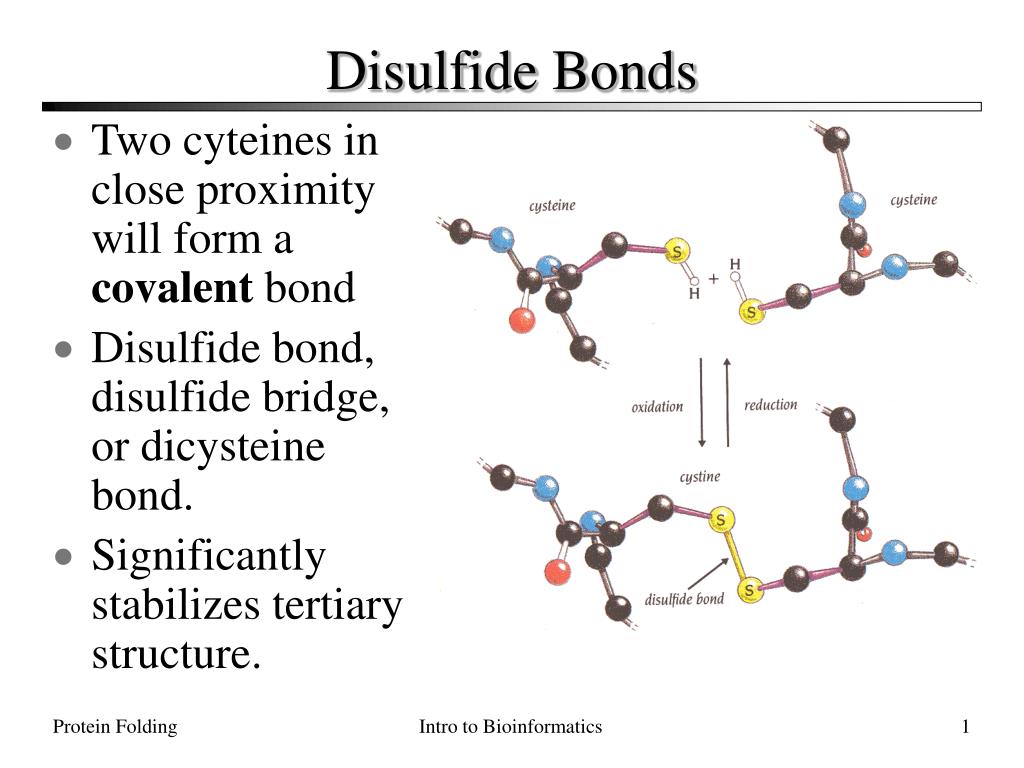
PPT Disulfide Bonds PowerPoint Presentation ID165240
Disulfide bond wikidoc
Disulfide bond wikidoc
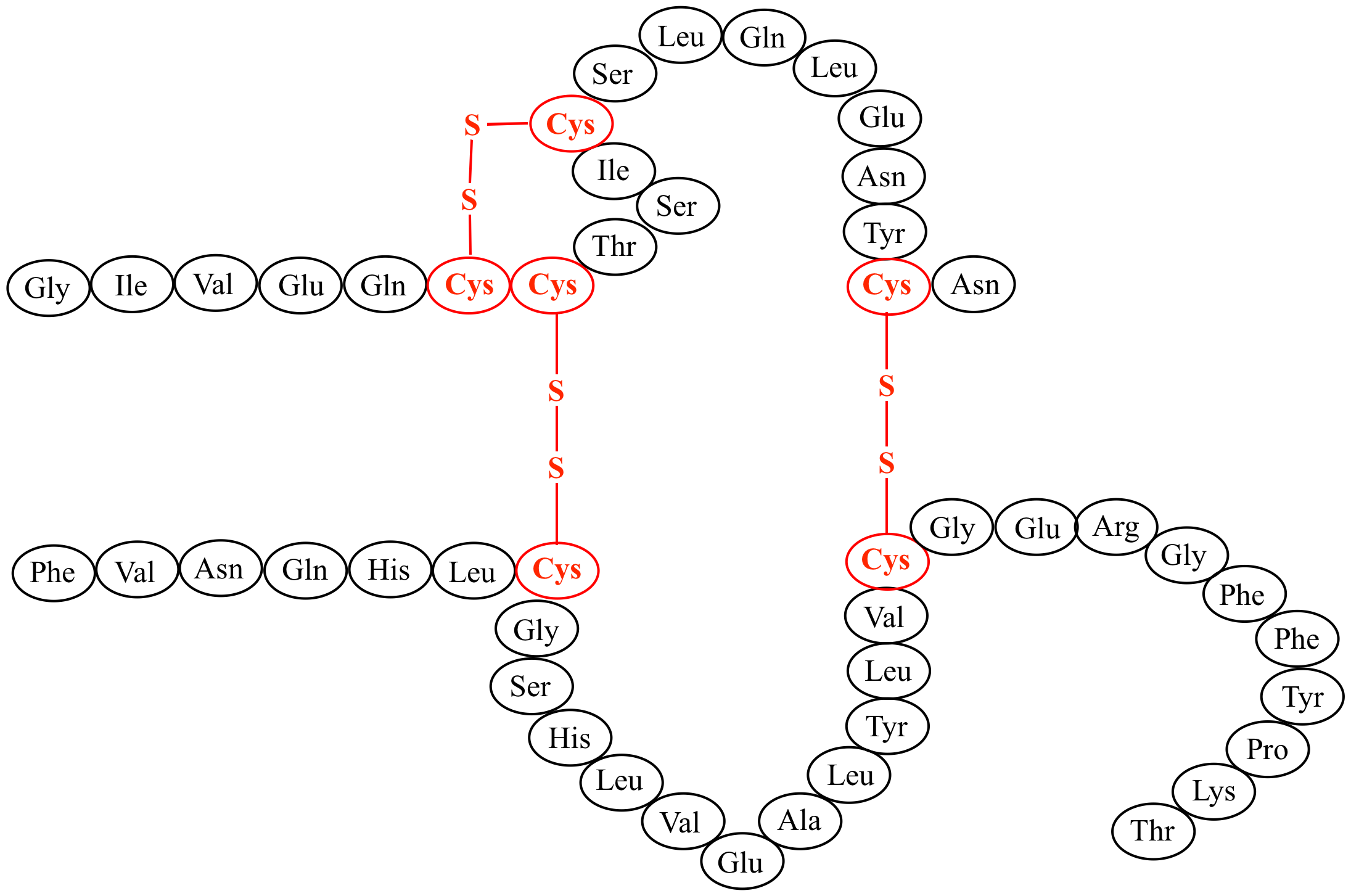
Along came a spider Digital World Biology
LabXchange
Mechanisms of cleavage of allosteric disulfide bonds. Disulfide bond
A disulfide bridge is an example of which type of bond? Select one a
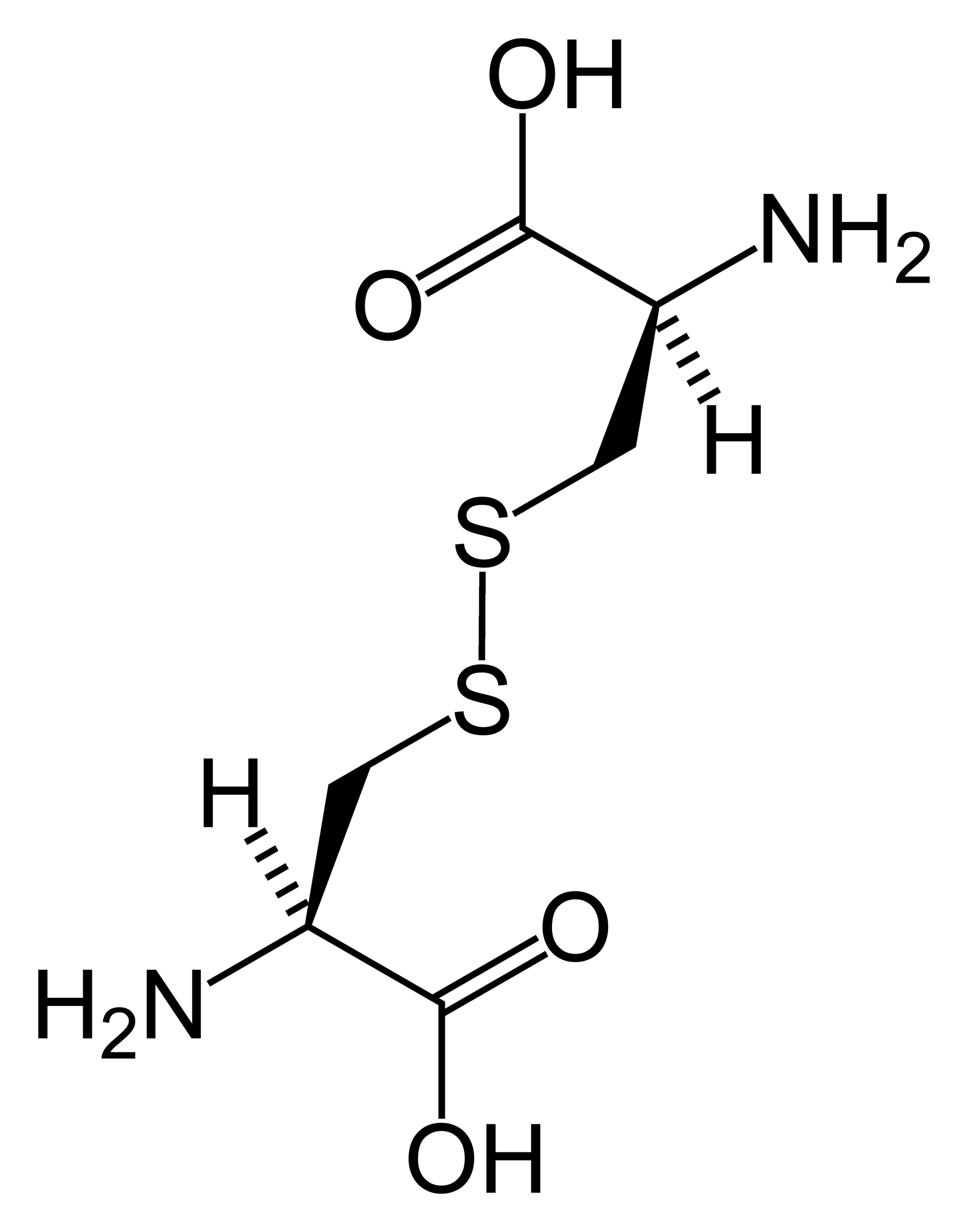
Illustrated Glossary of Organic Chemistry Disulfide bridge
Related Post: