Can Metals Form Covalent Bonds
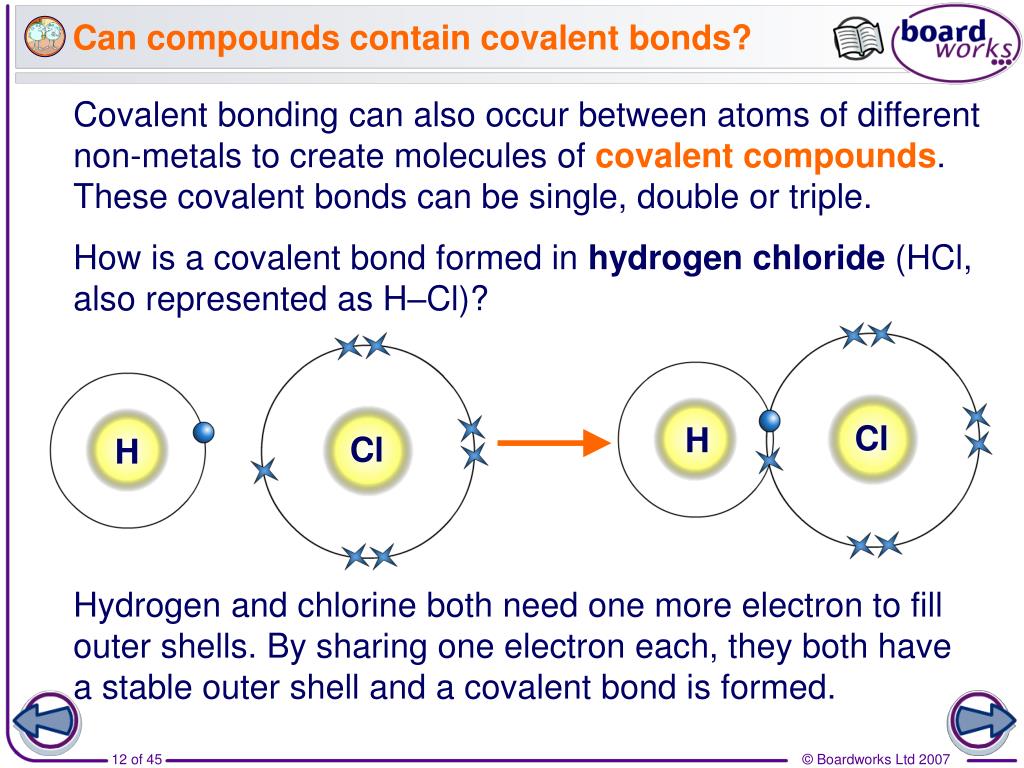
Can Metals Form Covalent Bonds - There are several examples such as kx2[rex2clx8]⋅2hx2o. For example, the hydrogen molecule, h 2, contains a covalent bond. Single and multiple covalent bonds. Then, there's hydrogen, a colorless and odorless gas. Web a metallic bond behaves more like one big molecule (except that unlike diamond or graphite, it’s malleable because there aren’t technically any covalent bonds forming a. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. But in another textbook i read that lithium,. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Single and multiple covalent bonds. Web metals simply do not hold on to electrons with enough strength to form much in the way of covalent bonds. An atom that shares one or more of its. Covalent bonding involves the sharing of electrons. Web ionic and covalent bonding. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. Web formation of covalent bonds. Metallic bonds exist in metal crystal lattices. For example, the hydrogen molecule, h 2, contains a covalent bond. Web science > ap®︎/college biology > chemistry of life > introduction to biological macromolecules. Web again, it's not true that metals don't form covalent bonds at all. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. For example, the hydrogen molecule, h 2, contains a covalent bond. Web ionic and covalent bonding. Is determined by the distance at which the lowest potential energy. Then, there's hydrogen, a colorless and odorless gas. Single and multiple covalent bonds. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. There are several examples such as kx2[rex2clx8]⋅2hx2o. There are primarily two forms of bonding that an atom can participate in: There are several examples such as kx2[rex2clx8]⋅2hx2o. Web a metallic bond behaves more like one big molecule (except that unlike diamond or graphite, it’s malleable because there aren’t technically any covalent bonds forming a. Web we would like to show you a description here but the site won’t allow us. Web formation of covalent bonds. Web again, it's not true. Web the answer is usually a metal. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. There are primarily two forms of bonding that an atom can participate in: For example, the hydrogen molecule, h 2, contains a covalent bond. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. The potential energy of two separate hydrogen atoms (right) decreases as. Fluorine and the other halogens in group 7a (17) have seven valence. Web science > ap®︎/college biology > chemistry of life > introduction to biological macromolecules. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. For example, the hydrogen molecule, h 2, contains a covalent bond. Web a metallic bond behaves more like one big molecule (except that unlike diamond or graphite, it’s malleable because there aren’t technically any covalent bonds forming a. But in another textbook i read that lithium,. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. The electrons involved are in. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web science > ap®︎/college biology > chemistry of life > introduction to biological macromolecules. The potential energy of two separate hydrogen atoms (right) decreases as. An atom that shares one or more of its. Web ionic and covalent bonding. But in another textbook i read that lithium,. There are several examples such as kx2[rex2clx8]⋅2hx2o. Web metals simply do not hold on to electrons with enough strength to form much in the way of covalent bonds. Covalent bonds involve the sharing of electron pairs between atoms. Web a metallic bond behaves more like one big molecule (except that unlike diamond. There are several examples such as kx2[rex2clx8]⋅2hx2o. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. The electrons involved are in the outer shells of the atoms. Web a metallic bond behaves more like one big molecule (except that unlike diamond or graphite, it’s malleable because there aren’t technically any covalent bonds forming a. The potential energy of two separate hydrogen atoms (right) decreases as. Web the answer is usually a metal. Web again, it's not true that metals don't form covalent bonds at all. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. Covalent bonding involves the sharing of electrons. An atom that shares one or more of its. Web my chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Neither atom is strong enough to attract electrons from the other. Is determined by the distance at which the lowest potential energy is achieved. One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. For a covalent bond to form, we need two atoms that both attract. Web we would like to show you a description here but the site won’t allow us. Covalent bonds involve the sharing of electron pairs between atoms. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. For example, the hydrogen molecule, h 2, contains a covalent bond.PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Ionic Bonding Presentation Chemistry
Examples of Covalent Bonds Several Examples of Covalent (molecular
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Covalent Bonding (Biology) — Definition & Role Expii
Metallic bond Properties, Examples, & Explanation Britannica
PPT Why do atoms form bonds? PowerPoint Presentation, free download
covalent bond Definition, Properties, Examples, & Facts Britannica
PPT Ionic and Covalent Compounds PowerPoint Presentation, free
Covalent Bond Biology Dictionary
Related Post:

.PNG)