Can Hf Form Hydrogen Bonds
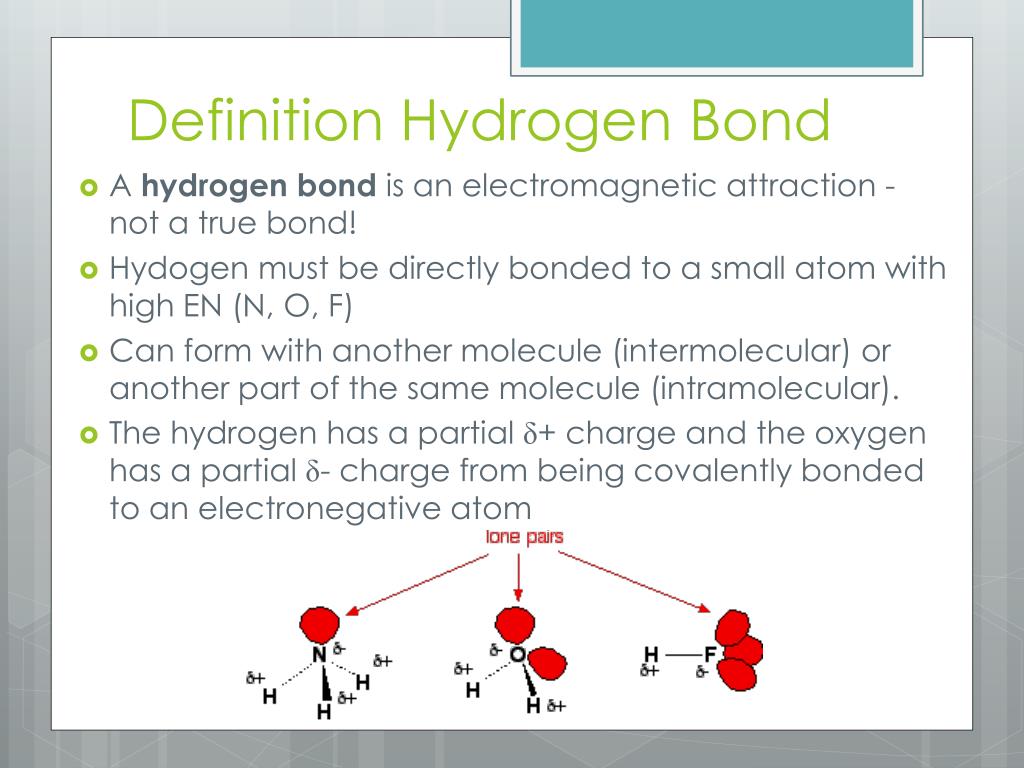
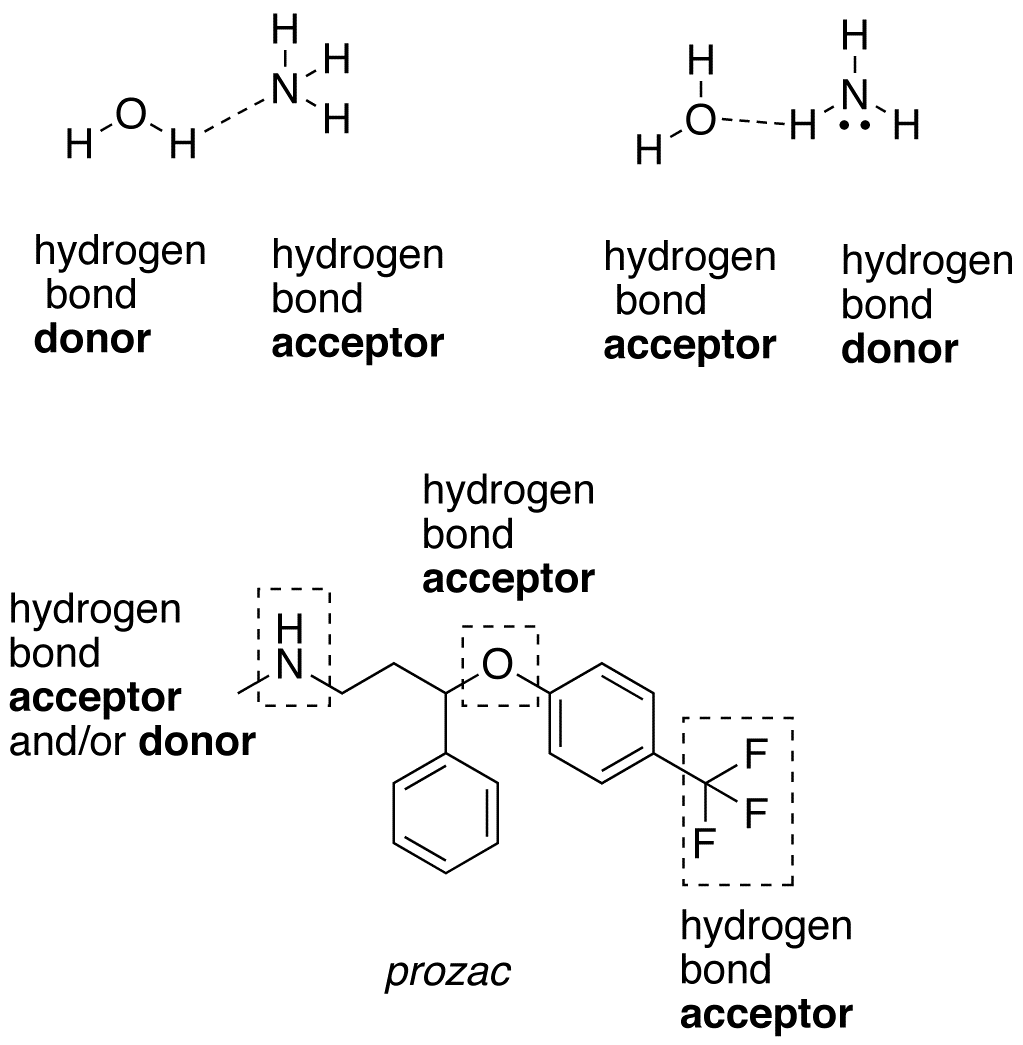
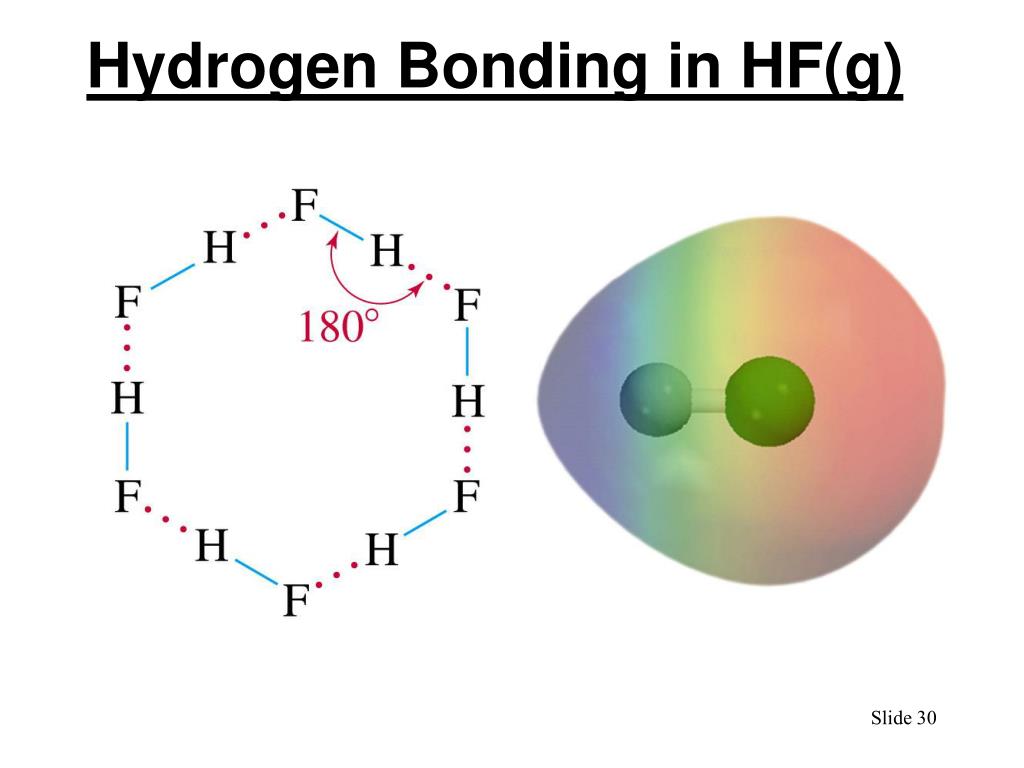
Can Hf Form Hydrogen Bonds - Water molecules are also attracted to other polar molecules. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: The higher the electronegativity, the. Furthermore, h2o h 2 o has a smaller molar mass. Web the conditions for hydrogen bonding are as follows: Web the hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as o , n , or f. Only the carbon atoms are. The molecule must contain a highly electronegative atom linked to the hydrogen atom. Here the distance between hydrogen. Web (hydrogen bond donor) second molecule has a lone pair of electrons on a small highly electronegative atom (n,o,f). These are relatively small atoms that can attach to hydrogen. Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond.. Furthermore, h2o h 2 o has a smaller molar mass. Such a bond is weaker than an ionic bond or. Web in hydrogen fluoride, the problem is a shortage of hydrogens. Also, unlike it has been said in the comments,. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. Web in hf each molecule got of hydrogen atom this can. Web we would like to show you a description here but the site won’t allow us. Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern: Hydrogen bonds exist when hydrogen is attached to a fluorine, oxygen, or nitrogen atom. On average, then, each molecule can only form one. Web hydrofluoric acid (hf): Web in hydrogen fluoride, the problem is a shortage of hydrogens. Web we would like to show you a description here but the site won’t allow us. Hydrogen bonds exist when hydrogen is attached to a fluorine, oxygen, or nitrogen atom. Web (hydrogen bond donor) second molecule has a lone pair of electrons on a small. These are relatively small atoms that can attach to hydrogen. This is a hydrogen bond. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Also, unlike it has been said in the comments,. Web the hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as o ,. In dimethyl ether, chx3ochx3 c h x 3 o c h x 3, all the hydrogen atoms are bonded to carbon in methyl groups. On average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving. Web the fairly positive hydrogen on one hf molecule will be attracted to one of these lone pairs. Web (hydrogen bond donor) second molecule has a lone pair of electrons on a small highly electronegative atom (n,o,f). This is a hydrogen bond. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web in hf each molecule got of hydrogen atom. This is a hydrogen bond. Only the carbon atoms are. Web in hf each molecule got of hydrogen atom this can submit a hydrogen bond, and go are three lone pairs of electronics on the fluorine atom. These are relatively small atoms that can attach to hydrogen. Web $\ce{hf}$ can not form two hydrogen bonds since the two positively charged. Web the hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as o , n , or f. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web $\ce{hf}$ can not form two hydrogen bonds since the two positively charged $\ce{h}$ atoms would repel. Web the hydrogen atoms involved in hydrogen bonding must be attached to electronegative atoms, such as o , n , or f. The molecule must contain a highly electronegative atom linked to the hydrogen atom. Web the conditions for hydrogen bonding are as follows: Water molecules are also attracted to other polar molecules. Web hydrofluoric acid (hf): Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web this is because h2o h 2 o, hf h f, and nh3 n h 3 all exhibit hydrogen bonding, whereas the others do not. Only the carbon atoms are. Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; On average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen and one involving. Furthermore, h2o h 2 o has a smaller molar mass. Web we would like to show you a description here but the site won’t allow us. Web in hydrogen fluoride, the problem is a shortage of hydrogens. Web in the cases of nh 3, h 2 o and hf there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. Hydrofluoric acid forms what is called a symmetric hydrogen bond, which is stronger than the regular hydrogen bond. Such a bond is weaker than an ionic bond or. Also, unlike it has been said in the comments,. Here the distance between hydrogen. Web hydrogen bonding between hf molecules is particularly evident in solid hf, where the atoms are arranged in a zigzag pattern:How do Hydrogen bonds form in H2O NH3 HF Hydrogen Bonding
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
(a) Hydrogen bonding patterns that describe the αand πconfigurations
Why does "HF" have a lower boiling point than water even though "F" is
hydrogen bond types Best Chemistry Blogs & Tutorials Digital
How can the hydrogen bonds in solid HF be best represented? ECHEMI
PPT Chapter 11 PowerPoint Presentation, free download ID5852920
What Intermolecular Forces Are Present In HF? Mastery Wiki
Hydrogen Bonding in Hydrogen fluoride (HF) YouTube
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
Related Post: