Can Co2 Form Hydrogen Bonds
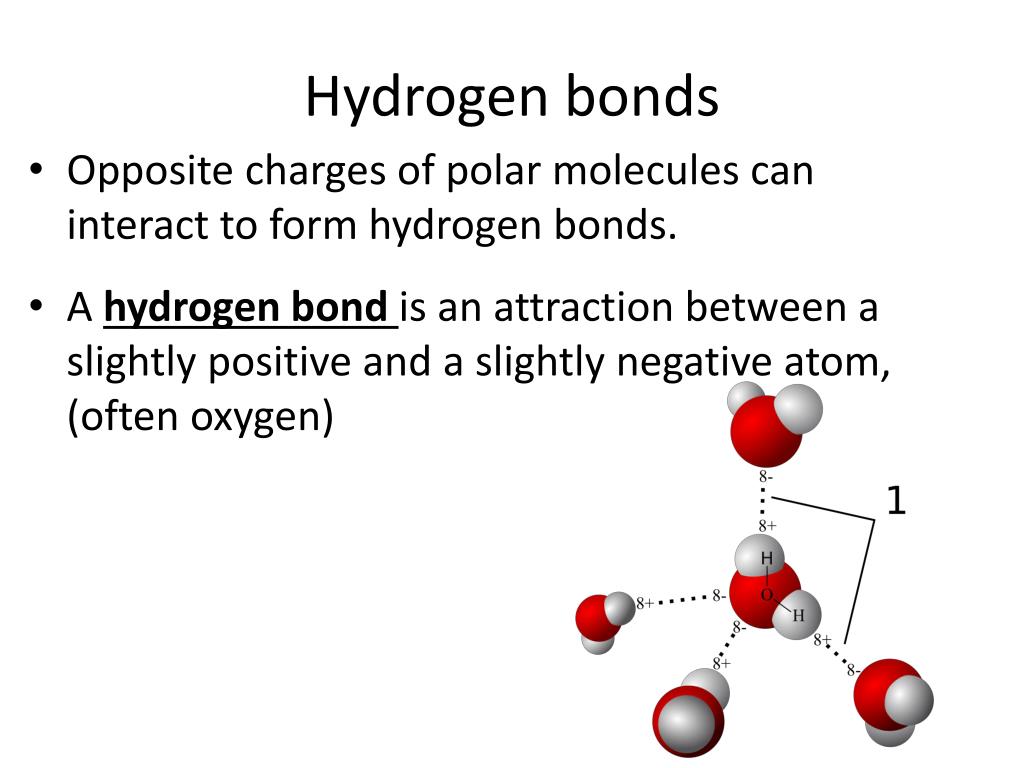
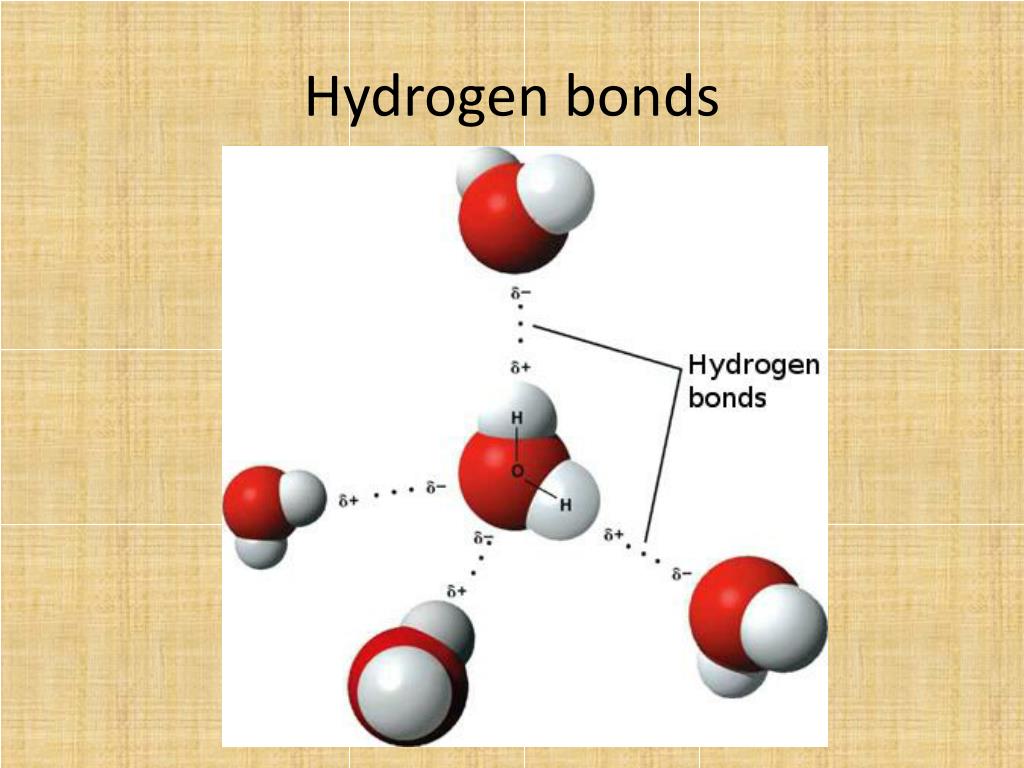
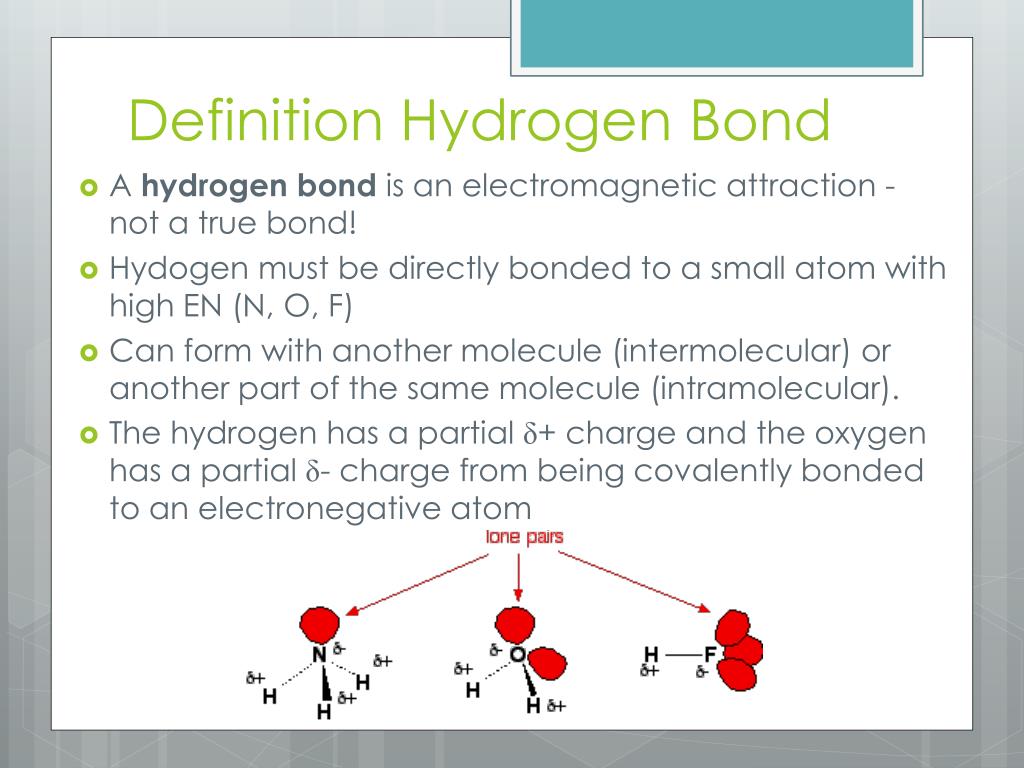
Can Co2 Form Hydrogen Bonds - Web hydrogen is the simplest, most abundant element on earth, accounting for 10% of a human's body weight, according to the u.s. I'm trying to model the mixture of. There is a double covalent bond between each of the carbon atoms and the. Web hydrogen bonds are formed between a slightly positive hydrogen atom and a slightly negative atom, usually oxygen or nitrogen. Web a hydrogen bond is an attraction between two atoms that already participate in other chemical bonds. Web the hydrogen is attached directly to one of the most electronegative elements, causing the hydrogen to acquire a significant amount of positive charge. Web 1 answer sorted by: Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web 1 answer sorted by: The majority dissociates into additional bicarbonate and free hydrogen. Web a hydrogen bond is an attraction between two atoms that already participate in other chemical bonds. This means that carbon dioxide is less soluble in water than polar molecules. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is. This means that carbon dioxide is less soluble in water than polar molecules. Web the hydrogen is attached directly to one of the most electronegative elements, causing the hydrogen to acquire a significant amount of positive charge. Web 1 answer sorted by: This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two. Web hydrogen bonds are formed between a slightly positive hydrogen atom and a slightly negative atom, usually oxygen or nitrogen. One big difference between the compounds is that sox2 s o x 2 is much more volatile, with a boiling point. Properties and bonding patterns of carbon atoms. Web very little of the extra carbon dioxide that is added into. The majority dissociates into additional bicarbonate and free hydrogen. Only one, the one at the very top which is attached to the highly. Thus, ethers containing up to 3 carbon. Web co2 can form hydrogen bonds with water, but its linear shape makes it a nonpolar molecule. 1 according to the this pubchem data table for the physical and chemical. Web hydrogen is the simplest, most abundant element on earth, accounting for 10% of a human's body weight, according to the u.s. Web co2 can form hydrogen bonds with water, but its linear shape makes it a nonpolar molecule. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared. Web basically, i want to know if molecular hydrogen (h 2 2) can form hydrogen bonds with other molecules (ch 4 4, co 2 2, n 2 2 ). Web the hydrogen producer must choose between the two credits on offer. Web 1 answer sorted by: Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? For instance, if. The biden administration announced plans on friday to award up to $7 billion to create seven regional hubs around the country that will make and use hydrogen, a. Web hydrogen is the simplest, most abundant element on earth, accounting for 10% of a human's body weight, according to the u.s. For instance, if you’ve used a pencil, you’ve seen. The. Web does hydrogen produce carbon dioxide when burned? Web hydrogen is the simplest, most abundant element on earth, accounting for 10% of a human's body weight, according to the u.s. Properties and bonding patterns of carbon atoms. One big difference between the compounds is that sox2 s o x 2 is much more volatile, with a boiling point. Web co2. This means that carbon dioxide is less soluble in water than polar molecules. Web does hydrogen produce carbon dioxide when burned? Web the reason co2 can't hydrogen bond is that there are no lone pairs of electrons. Web basically, i want to know if molecular hydrogen (h 2 2) can form hydrogen bonds with other molecules (ch 4 4, co. This means that carbon dioxide is less soluble in water than polar. Web 1 answer sorted by: Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web basically, i want to know if molecular hydrogen (h 2 2) can form hydrogen bonds with other molecules (ch 4 4, co. Web co2 can form hydrogen bonds with water, but its linear shape makes it a nonpolar molecule. Web 1 answer sorted by: Web co2 can form hydrogen bonds with water, but its linear shape makes it a nonpolar molecule. 8 there are a couple different forces at play here. Thus, ethers containing up to 3 carbon. Web hydrogen bonds are formed between a slightly positive hydrogen atom and a slightly negative atom, usually oxygen or nitrogen. One big difference between the compounds is that sox2 s o x 2 is much more volatile, with a boiling point. Web hydrogen is the simplest, most abundant element on earth, accounting for 10% of a human's body weight, according to the u.s. Web basically, i want to know if molecular hydrogen (h 2 2) can form hydrogen bonds with other molecules (ch 4 4, co 2 2, n 2 2 ). Web hydrogen bonds can occur within one single molecule, between two like molecules, or between two unlike molecules. I'm trying to model the mixture of. Web the hydrogen is attached directly to one of the most electronegative elements, causing the hydrogen to acquire a significant amount of positive charge. This means that carbon dioxide is less soluble in water than polar molecules. In cells, these bonds are primarily formed. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. For instance, if you’ve used a pencil, you’ve seen. This means that carbon dioxide is less soluble in water than polar. Web the hydrogen producer must choose between the two credits on offer. Web very little of the extra carbon dioxide that is added into the ocean remains as dissolved carbon dioxide. Web expert answer 100% (32 ratings) transcribed image text:PPT Properties of Water PowerPoint Presentation, free download ID
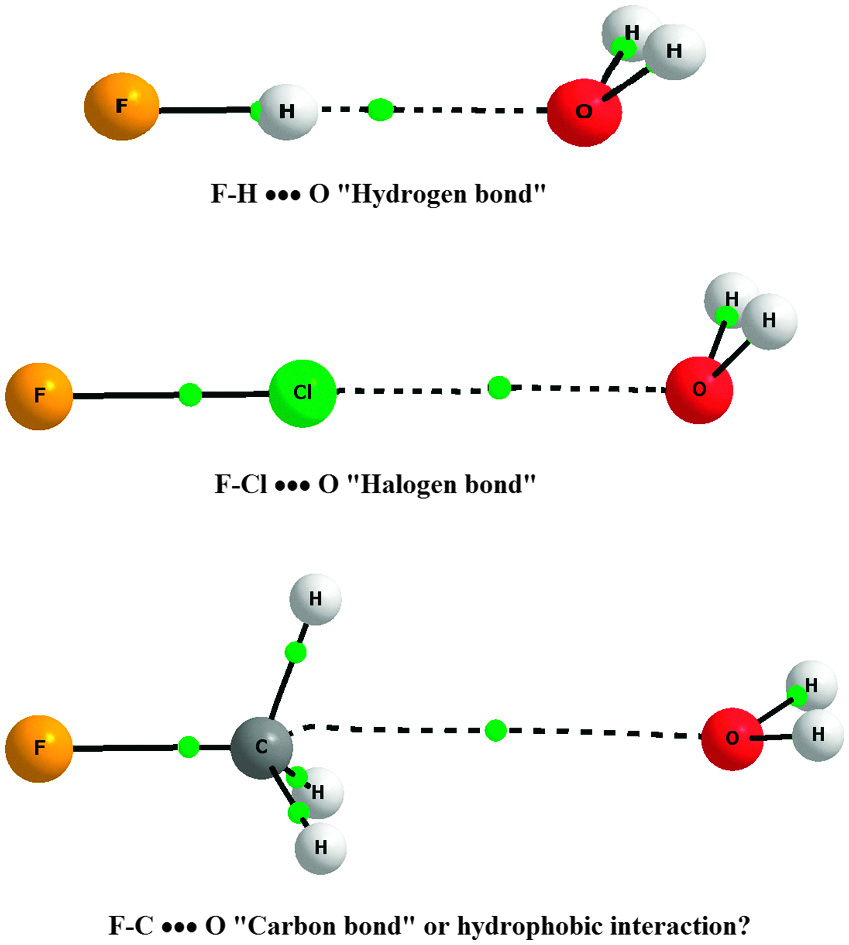
Exploring the carbon bond PCCP Blog
The top panel in this figure shows two hydrogen atoms sharing two
PPT Chemistry of Life PowerPoint Presentation, free download ID2666943
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Four covalent bonds. Carbon has four valence electrons and here a
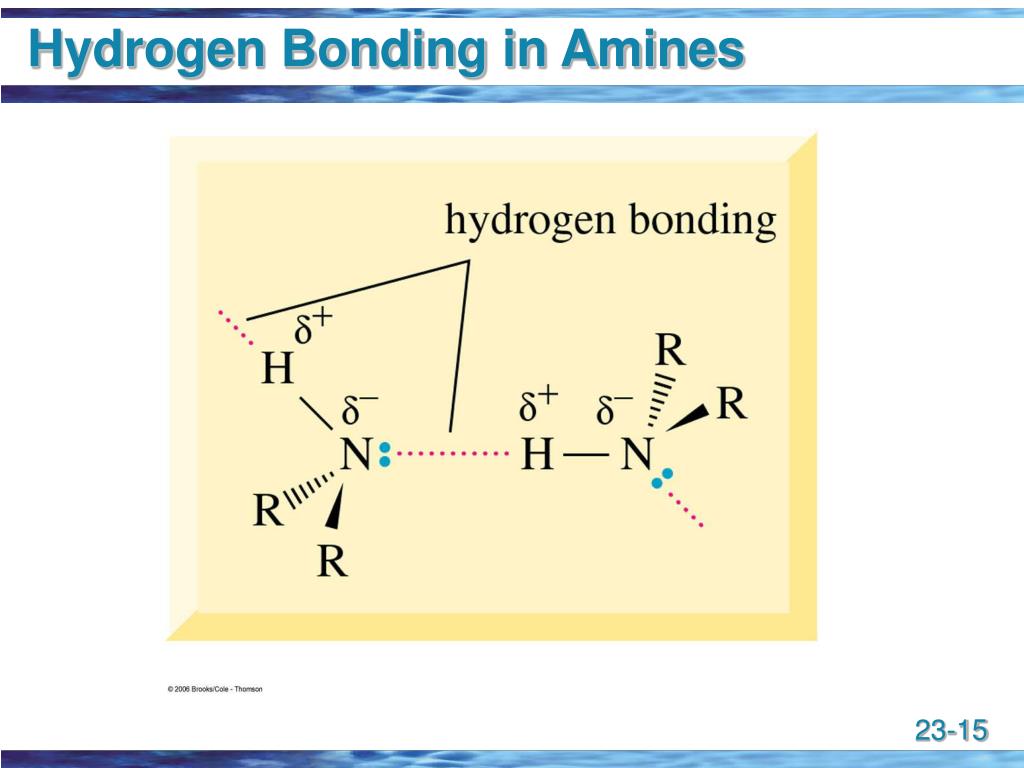
PPT Amines PowerPoint Presentation, free download ID149830
9 Hydrogen Bond Examples in Real Life StudiousGuy
PPT Covalent Compounds PowerPoint Presentation, free download ID
PPT Biochemistry PowerPoint Presentation, free download ID89333
Related Post: