Can C-H Form Hydrogen Bonds
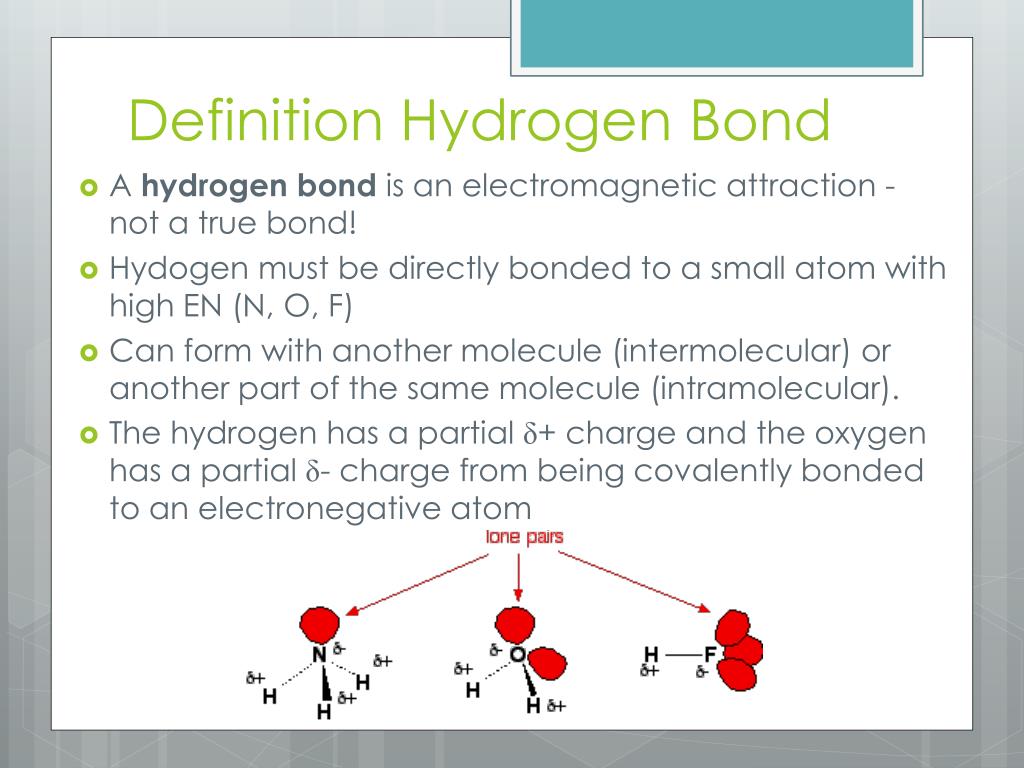
Can C-H Form Hydrogen Bonds - Answer only one, the one at the very top which is attached to the highly electrongative. Web in this explainer, we will learn how to describe and explain hydrogen bonding and the effect it has on the physical properties of molecules. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Electronegativity effects (section 2), steric effects (section 3),. Become a study.com member to unlock this answer! Web chemistry of life > structure of water and hydrogen bonding. Hydrogen bonds form between base pairs. Intramolecular hydrogen bonding occurs between hydrogen and oxygen. Web acetylacetone (c 5 h 8 o 2 ): Web hydrogen bonds may form between atoms within a molecule or between two separate molecules. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Ch4 cannot form hydrogen bonds. A hydrogen bond is weaker than an ionic bond or a covalent. Web hydrogen bonds can occur within one single molecule, between two like molecules, or between two unlike molecules. The structure of water molecules and how they can interact to form. Web acetylacetone (c 5 h 8 o 2 ): Web chemistry of life > structure of water and. A hydrogen bond is weaker than an ionic bond or a covalent. Web acetylacetone (c 5 h 8 o 2 ): Answer only one, the one at the very top which is attached to the highly electrongative. Hydrogen bonds form between base pairs. Such a bond is weaker than an ionic bond. Answer only one, the one at the very top which is attached to the highly electrongative. So yes, we can have hydrogen bonding between one h2o. The structure of water molecules and how they can interact to form. Intramolecular hydrogen bonding occurs between hydrogen and oxygen. Web hydrogen bonds can form between different molecules, as long as one molecule has. Web hydrogen bonds can occur within one single molecule, between two like molecules, or between two unlike molecules. A hydrogen bond is weaker than an ionic bond or a covalent. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Answer only one, the one at the very top. Answer only one, the one at the very top which is attached to the highly electrongative. Electronegativity effects (section 2), steric effects (section 3),. A hydrogen bond is weaker than an ionic bond or a covalent. There are exactly the right numbers of + hydrogens. Intramolecular hydrogen bonding occurs between hydrogen and oxygen. Ch4 cannot form hydrogen bonds. Web acetylacetone (c 5 h 8 o 2 ): Such a bond is weaker than an ionic bond. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Intramolecular hydrogen bonding occurs between hydrogen and oxygen. Answer only one, the one at the very top which is attached to the highly electrongative. Web hydrogen bonds can occur within one single molecule, between two like molecules, or between two unlike molecules. Web in this explainer, we will learn how to describe and explain hydrogen bonding and the effect it has on the physical properties of molecules. Web. Electronegativity effects (section 2), steric effects (section 3),. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Intramolecular hydrogen bonding occurs between hydrogen and oxygen. So yes, we can have hydrogen bonding between one h2o. Hydrogen bonds form between base pairs. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web hydrogen bonds may form between atoms within a molecule or between two separate molecules. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. There are exactly the right numbers of + hydrogens. So yes,. So yes, we can have hydrogen bonding between one h2o. Web this review presents an overview of hydrogen bond design principles, based on five structural features: Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Hydrogen bonds form between base pairs. Electronegativity effects (section 2), steric effects (section 3),. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? A hydrogen bond is weaker than an ionic bond or a covalent. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web chemistry of life > structure of water and hydrogen bonding. Web hydrogen bonds can occur within one single molecule, between two like molecules, or between two unlike molecules. Ch4 cannot form hydrogen bonds. Become a study.com member to unlock this answer! Such a bond is weaker than an ionic bond. Web hydrogen bonds may form between atoms within a molecule or between two separate molecules. The structure of water molecules and how they can interact to form. Answer only one, the one at the very top which is attached to the highly electrongative. Intramolecular hydrogen bonding occurs between hydrogen and oxygen. Web in this explainer, we will learn how to describe and explain hydrogen bonding and the effect it has on the physical properties of molecules. Web acetylacetone (c 5 h 8 o 2 ):PPT hydrogen bond PowerPoint Presentation, free download ID4524678
Hydrogen Bonding Definition, Example, Types, Question Embibe
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Hydrogen Bonds — Overview & Examples Expii
9 Hydrogen Bond Examples in Real Life StudiousGuy
Rules for Hydrogen bonding? Mcat
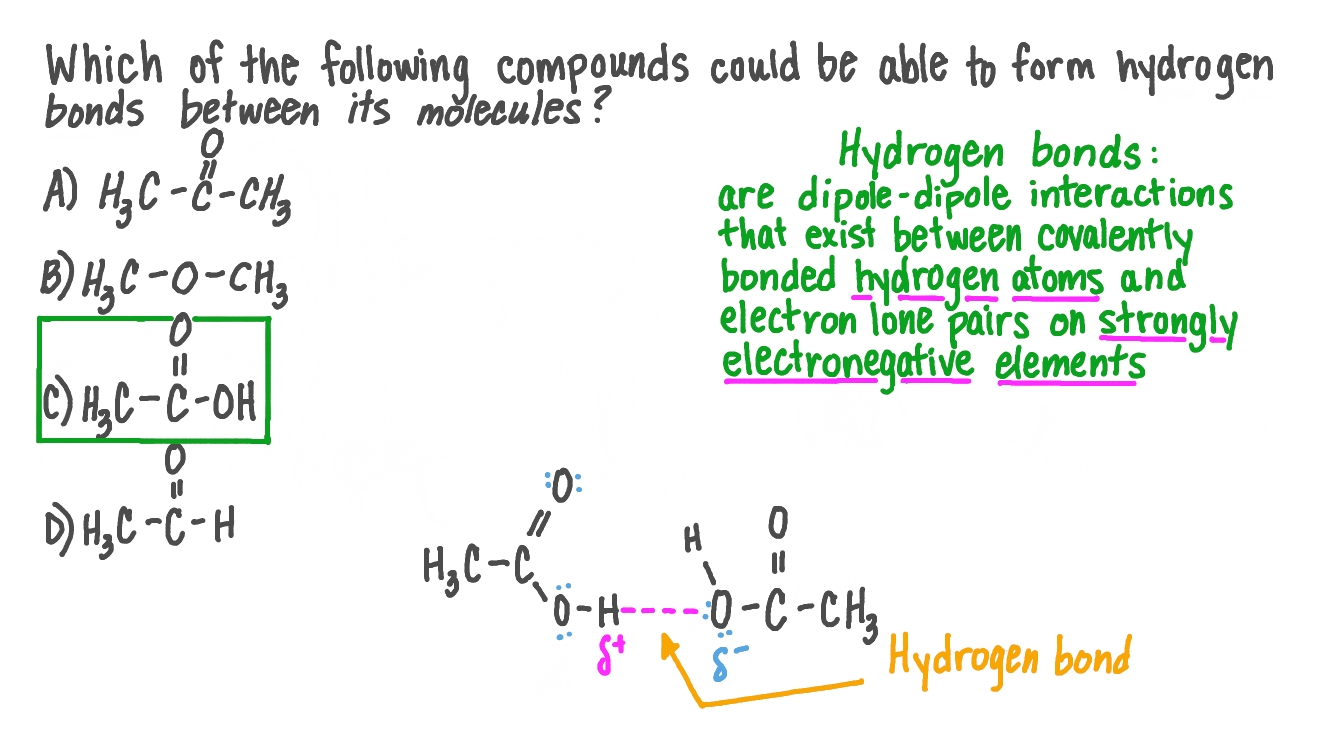
Question Video Identifying Which Compound Could Form Hydrogen Bonds
(a) Hydrogen bonding patterns that describe the αand πconfigurations
Hydrogen Bonding American Chemical Society
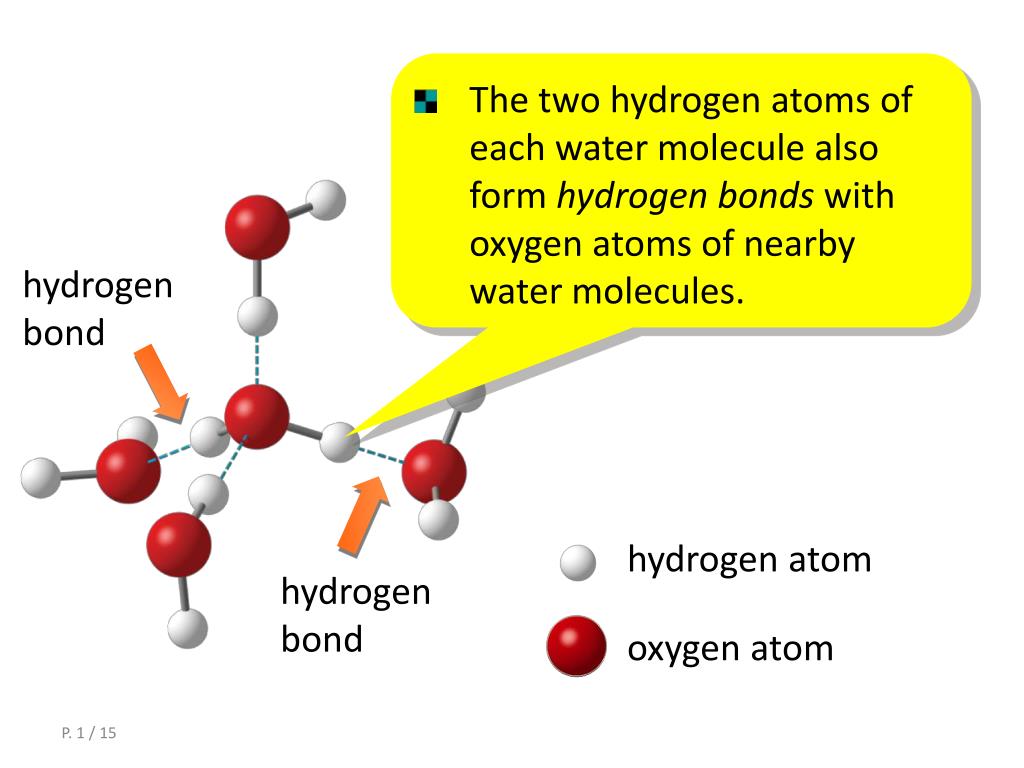
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Related Post: