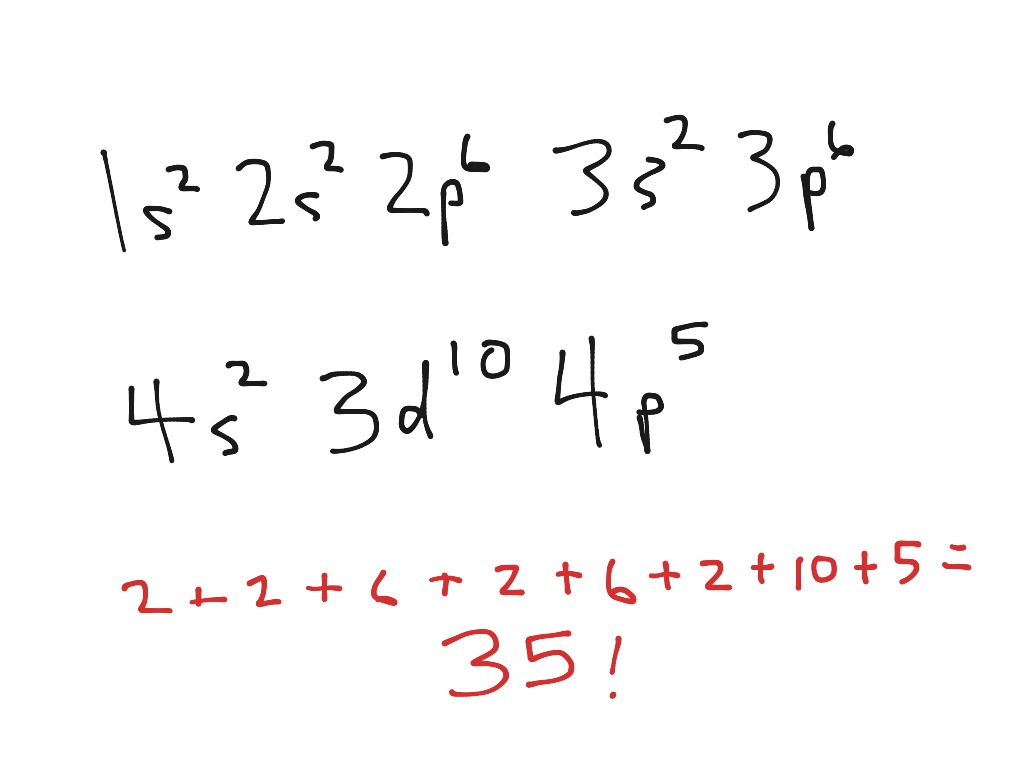Br Electron Configuration Long Form
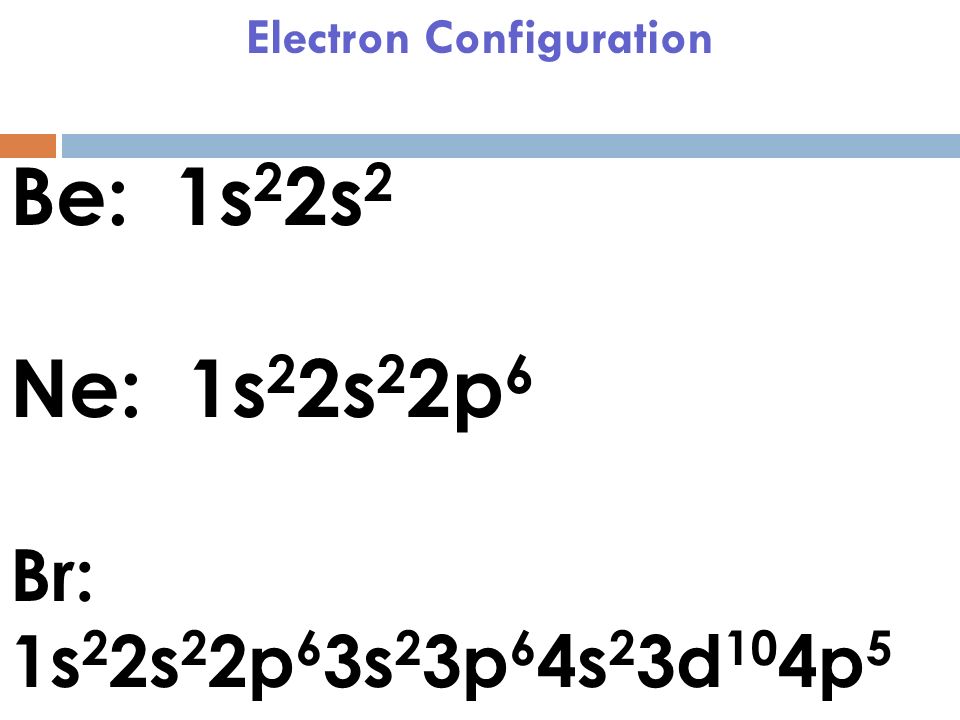
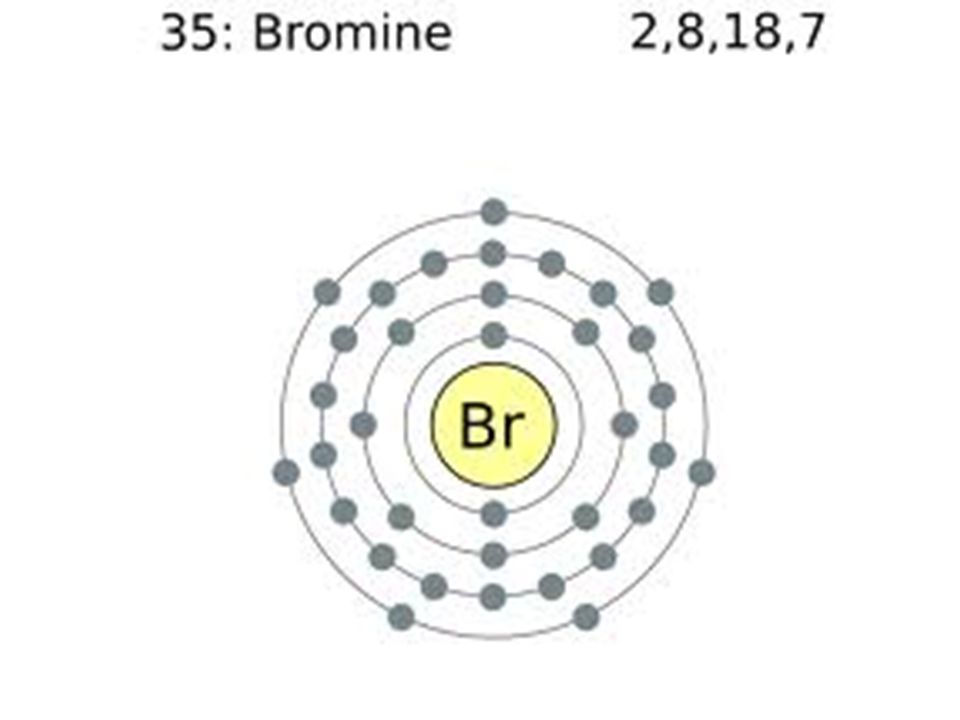
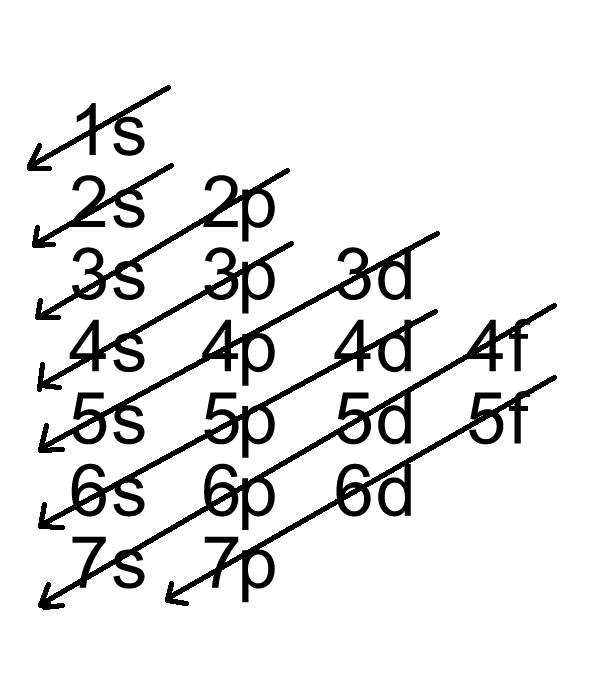
Br Electron Configuration Long Form - $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ because it have one more electron than bromine, which ends its electronic configuration with $\mathrm{4p^5}$. Web to help describe the appropriate notation for electron configuration, it is best to do so through example. For example, the ground state electron configuration of nitrogen (1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1 s 2 2 s 2 2 p 3) indicates that it has 3 3 3 electrons occupying the 2 p 2 \rm p 2 p orbital. Electron configuration of carbon (c) [he] 2s 2 2p 2: Web what is the electronic configuration of bromine? Bromine belongs to group 17 which is known as halogens. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5. 1s 2 2s 2 2p 6 3s 2 3p 1. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Web electron configuration of bromine is [ar] 3d10 4s2 4p5. 265.90 k, 5.8 kpa : Electronic configuration of group 17: Web write out the full electron configuration for each of the following atoms and for the monatomic ion found in binary ionic compounds containing. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. Nevertheless, check. 1s 2 2s 2 2p 1: 1s 2 2s 2 2p 3: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Web the electron configuration of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 or [ ar] 4s 2 3d 10 4p 5. The periodic table is a tabular. Melting point (br 2) 265.8 k (−7.2 °c, 19 °f) boiling point (br 2) 332.0 k (58.8 °c, 137.8 °f) density (near r.t.) br 2, liquid: A neutral oxygen atom has 8 electrons, so the orbitals that will contain electrons are the 1s, 2s and 2p. Electronic configuration of the bromine atom in ascending order of orbital energies: Web write. Web write out the full electron configuration for each of the following atoms and for the monatomic ion found in binary ionic compounds containing the element: Web some are hard to memorise (or predict), so what is the electron configuration of an atom of br? The elements of group 17 have seven electrons in their outermost shell. Electron configuration of. Melting point (br 2) 265.8 k (−7.2 °c, 19 °f) boiling point (br 2) 332.0 k (58.8 °c, 137.8 °f) density (near r.t.) br 2, liquid: 1s 2 2s 2 2p 6 3s 2 3p 1. Web electron configuration of beryllium (be) [he] 2s 2: Web electron configuration 3d 10 4s 2 4p 5: [ar] 3d 10 4s 2 4p. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. Web this makes the shorthand electron configuration for bromine [ar]4s23d104p5. Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. Electron configuration of. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below to fill the subshells in order of the diagonal lines. Electron configuration of carbon (c) [he] 2s 2 2p 2: Because it has no unpaired electrons, it is. 265.90 k, 5.8 kpa : The last example is an orbital diagram. Because it has no unpaired electrons, it is. Web to begin with, bromine (br) has an electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Electron configuration of boron (b) [he] 2s 2 2p 1: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. 1s 2 2s 2 2p 2: 1s 2 2s 2 2p 6. Web electron configuration of bromine is [ar] 3d10 4s2 4p5. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. 1s 2 2s 2 2p 1: Use a chart such as the one below to fill the subshells in order of the diagonal lines. Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. In the case of bromine the abbreviated electron configuration is [ar] 3d10 4s2 4p5. 1s 2 2s 2 2p 1: Nevertheless, check the complete configuration and other interesting facts about bromine that most people don't know. 1s 2 2s 2 2p 6 3s 2 3p 1. Following hydrogen is the noble gas helium, which has an atomic number of 2. The last example is an orbital diagram. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. 1s 2 2s 2 2p 2: Because it has no unpaired electrons, it is. Web the electron configuration and the orbital diagram are: Web this makes the shorthand electron configuration for bromine [ar]4s23d104p5. ← electronic configurations of elements. A neutral oxygen atom has 8 electrons, so the orbitals that will contain electrons are the 1s, 2s and 2p. Bromine belongs to group 17 which is known as halogens. Web electron configuration of beryllium (be) [he] 2s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5. 265.90 k, 5.8 kpa : This can be shortened to [ar]4s23d104p5.Electron Configuration of Bromine, Br YouTube
Atom Diagrams Electron Configurations of the Elements
Show The Orbital Filling Diagram For Br Bromine Free Wiring Diagram
Excited State Electron Configuration of Bromine
How Do We Find The Electron Configuration For Bromine Dynamic
Electron Configuration of Br Science, Chemistry, Elements ShowMe
How Can We Find A Electron Configuration For Bromine (Br)
Load Wiring Methane Electron Dot Diagram
Bromine Electron Configuration (Br) with Orbital Diagram
Same Electron Configuration as the Bromide Ion Br Brodsky Muchey
Related Post:

:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg)