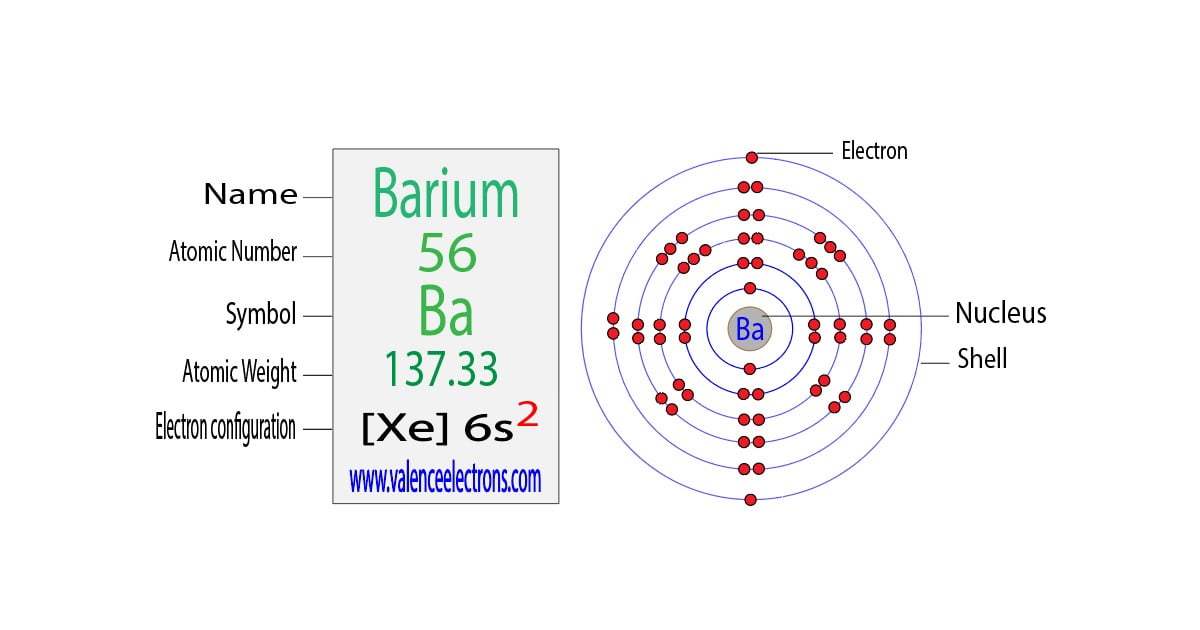
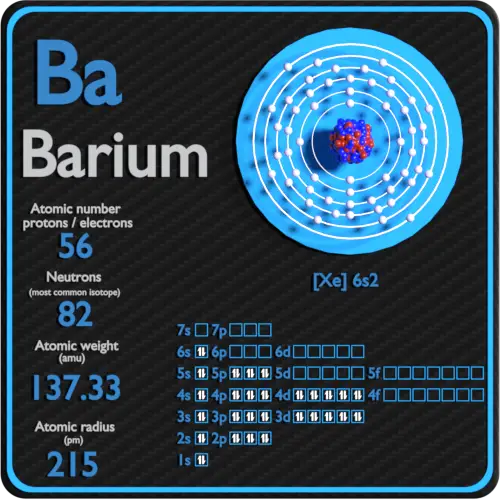
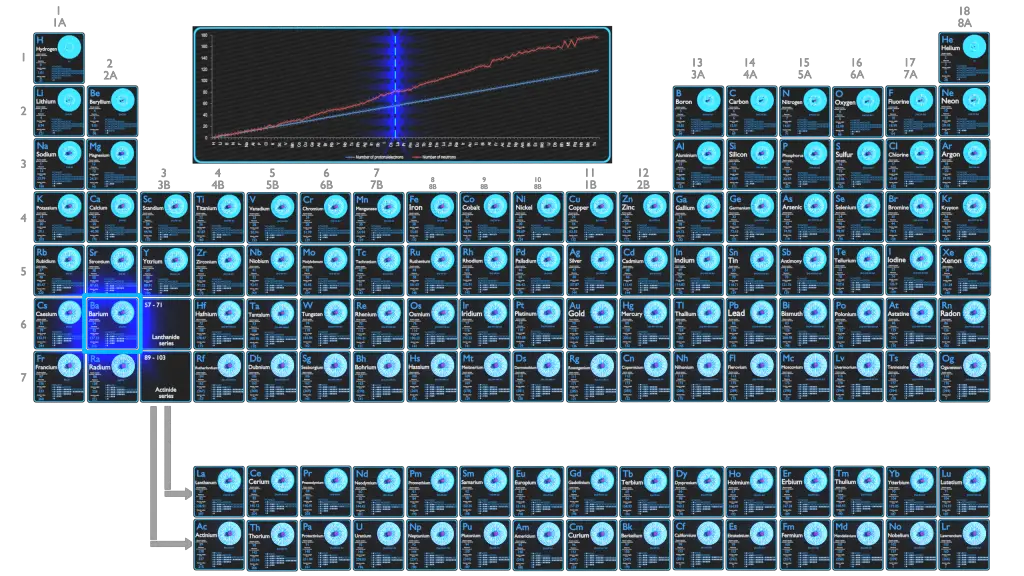
Barium Electron Configuration Long Form
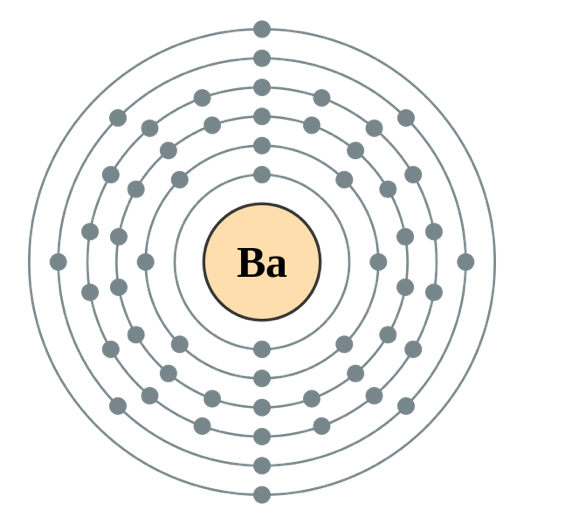
Barium Electron Configuration Long Form - Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. 30k views 3 years ago. A representation of the atomic spectrum of. Web the atomic number of barium is 56 and it belongs to the sixth period and the second group of the periodic table. 2118 k (1845 °c, 3353 °f) density (near r.t.) 3.51 g/cm 3: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. This page shows the electron configurations of the neutral gaseous atoms in their ground states. Web the electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 4s2 3d104p6 4d10 5s2 5p6 6s2. A barium atom gives away two electrons, to become a noble gas configuration. The commonly used long form of the periodic table is designed to emphasize electron configurations.since it is the outermost (valence) electrons which are primarily involved in chemical interactions between atoms, the last electron added to an atom in the building. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 5d 1 6s 2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web in order to write the i electron configuration we first need to know the number of electrons for the barium (ba) atom.. 0.97 (allrod rochow) heat of fusion: The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. Web electron configuration 6s 2: So, the number of electrons are 56. This page shows the electron configurations of the neutral gaseous atoms in their ground states. Web electron configuration of barium (ba) [xe] 6s 2. Web electron configuration 6s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 2. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. 30k views 3. Web electron configuration 6s 2: Web barium atoms have 56 electrons and the shell structure is 2.8.18.18.8.2. Electron configuration of lanthanum (la) [xe] 5d 1 6s 2. List of unique identifiers for barium in various chemical registry databases. When we write the configuration,. A representation of the atomic spectrum of. To find out the valence electrons of barium, you have to see the position of barium in the periodic table. Web in order to write the i electron configuration we first need to know the number of electrons for the barium (ba) atom. 2, 8, 18, 18, 9, 2. Web the electron configuration. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 5d 1 6s 2. This electron configuration shows that barium ion(ba 2+ ) has five shells and the last shell has eight electrons. Web electron configuration 6s 2: The commonly used long form of the periodic table is. A representation of the atomic spectrum of. The electronic configuration of barium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. 0.97 (allrod. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. When we write the configuration,. The electron configuration is the standard notation used to describe the electronic structure of an atom. A representation of the atomic spectrum of. So, the number of electrons are 56. Electron configuration of lanthanum (la) [xe] 5d 1 6s 2. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from. This electron configuration shows that barium ion(ba 2+ ) has five shells and the last shell has eight electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. The. 6s2 and the term symbol is 1s0. When liquid (at m.p.) 3.338 g/cm 3 : Web the full electron (s,p,d,f) configuration would be: In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from. As the atomic number is equal to the number of electrons present in a neutral element. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. Web electron configuration 6s 2: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^6 6s^2 however, to simplify this, noble gas or core notation can be used. Web the long form electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. 1000 k (727 °c, 1341 °f) boiling point: A barium atom gives away two electrons, to become a noble gas configuration. The atomic mass of barium is 137.33 u and its density is 3.51 g/cm 3, which is quite dense among all the other alkaline earth metals on the periodic table. Web barium is a soft metal having silvery grey metallic lustre with a pale yellow tint. 2, 8, 18, 18, 9, 2. 6s2 and the term symbol is 1s0. Ba 2+ electron configuration (barium ion) wayne breslyn. 30k views 3 years ago. Web the full electron (s,p,d,f) configuration would be: List of unique identifiers for barium in various chemical registry databases. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 2. When liquid (at m.p.) 3.338 g/cm 3 : From the above image, you can see that the barium (ba) is present in the group 2 of periodic table. The electronic configuration of barium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6. The kossel shell structure of barium.Complete Electron Configuration for Barium (Ba, Ba2+ ion)
Barium Periodic Table and Atomic Properties
Symbol and electron diagram for barium Royalty Free Vector
Barium(Ba) electron configuration and orbital diagram (2022)
Electron Configuration of Barium Ba Lesson YouTube
A stepbystep description of how to write the electron configuration
Barium Protons Neutrons Electrons Electron Configuration
56. Barium The Quantum Bicycle Society
Orbital Diagram For Barium
Barium Adopt an Element Screen 3 on FlowVella Presentation
Related Post: